Abstract
Purpose
To compare the changes in subfoveal choroidal thickness between intravitreal aflibercept and ranibizumab injection in wet age-related macular degeneration (AMD).
Methods
Fifty patients with wet AMD patients who were treated with aflibercpet or ranibizumab were evaluated retrospectively. All patients were treated with pro re nata after 3 consecutive monthly injections and were followed up for at least 6 months. We measured subfoveal choroidal thickness (SFCT) using enhanced depth imaging spectral-domain optical coherence tomography before the first injection and 1, 2, 3, and 6 months after initial injection.
Results
The SFCT measures before initial injection and 1, 2, 3, and 6 months after initial injection were 244.94 ± 103.77 μ m, 219.04 ± 95.89 μ m, 208.74 ± 91.03 μ m, 203.64 ± 91.35 μ m, and 226.98 ± 96.79 μ m in the aflibercept group (25 eyes) and 222.68 ± 102.04 μ m, 210.23 ± 95.91 μ m, 203.66 ± 99.39 μ m, 197.27 ± 100.25 μ m, and 210.32 ± 111.86 μ m in the ranibizumab group (28 eyes). Mean change in SFCT was greater in the aflibercept group at 1 month, 2 months, and 3 months after initial injection ( p < 0.05), but there was no significant difference in the mean change in SFCT between the two groups at 6 months after initial in-jection ( p > 0.05).
References
1. Augood C, Fletcher A, Bentham G. . Methods for a pop-ulation-based study of the prevalence of and risk factors for age-re-lated maculopathy and macular degeneration in elderly European populations: the EUREYE study. Ophthalmic Epidemiol. 2004; 11:117–29.

2. Friedman DS, O'Colmain BJ, Muñoz B. . Prevalence of age-re-lated macular degeneration in the United States. Arch Ophthalmol. 2004; 122:564–72.

3. Park SJ, Lee JH, Woo SJ. . Age-related macular degeneration: prevalence and risk factors from Korean National Health and Nutrition Examination Survey, 2008 through 2011. Ophthalmology. 2014; 121:1756–65.
4. Rosenfeld PJ, Brown DM, Heier JS. . Ranibizumab for neo-vascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–31.

5. Brown DM, Kaiser PK, Michels M. . Ranibizumab versus ver-teporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–44.

6. Avery RL, Pieramici DJ, Rabena MD. . Intravitreal bev-acizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006; 113:363–72.e5.

7. Folk JC, Stone EM. Ranibizumab therapy for neovascular age-re-lated macular degeneration. N Engl J Med. 2010; 363:1648–55.

8. Lanzetta P, Mitchell P, Wolf S, Veritti D. Different antivascular en-dothelial growth factor treatments and regimens and their out-comes in neovascular age-related macular degeneration: a liter-ature review. Br J Ophthalmol. 2013; 97:1497–507.

9. Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group, Martin DF, Maguire MG. . Ranibizumab and bevacizumab for treatment of neovascular age-re-lated macular degeneration: two-year results. Ophthalmology. 2012; 119:1388–98.
10. Heier JS, Brown DM, Chong V. . Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012; 119:2537–48.

11. Blaauwgeers HG, Holtkamp GM, Rutten H. . Polarized vas-cular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic para-crine relation. Am J Pathol. 1999; 155:421–8.

12. Saint-Geniez M, Kurihara T, Sekiyama E. . An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci U S A. 2009; 106:18751–6.

13. Yamazaki T, Koizumi H, Yamagishi T, Kinoshita S. Subfoveal cho-roidal thickness after ranibizumab therapy for neovascular age-re-lated macular degeneration: 12-month results. Ophthalmology. 2012; 119:1621–7.

14. Koizumi H, Kano M, Yamamoto A. . Short-term changes in choroidal thickness after aflibercept therapy for neovascular age-related macular degeneration. Am J Ophthalmol. 2015; 159:627–33.

15. Kim JH, Lee TG, Chang YS. . Short-term choroidal thickness changes in patients treated with either ranibizumab or aflibercept: a comparative study. Br J Ophthalmol. 2016; 100:1634–9.

16. Yun C, Oh J, Ahn J. . Comparison of intravitreal aflibercept and ranibizumab injections on subfoveal and peripapillary choroi-dal thickness in eyes with neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2016; 254:1693–702.

17. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004; 25:581–611.

18. Stewart MW, Rosenfeld PJ. Predicted biological activity of intra-vitreal VEGF Trap. Br J Ophthalmol. 2008; 92:667–8.

19. Chappelow AV, Kaiser PK. Neovascular age-related macular de-generation: potential therapies. Drugs. 2008; 68:1029–36.
20. Rudge JS, Thurston G, Davis S. . VEGF trap as a novel anti-angiogenic treatment currently in clinical trials for cancer and eye diseases, and VelociGene- based discovery of the next generation of angiogenesis targets. Cold Spring Harb Symp Quant Biol. 2005; 70:411–8.
21. Papadopoulos N, Martin J, Ruan Q. . Binding and neutraliza-tion of vascular endothelial growth factor (VEGF) and related li-gands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012; 15:171–85.

22. Linsenmeier RA, Padnick-Silver L.Metabolic dependence of pho-toreceptors on the choroid in the normal and detached retina. Invest Ophthalmol Vis Sci. 2000; 41:3117–23.
23. Ardeljan D, Chan CC. Aging is not a disease: distinguishing age-related macular degeneration from aging. Prog Retin Eye Res. 2013; 37:68–89.

24. Julien S, Biesemeier A, Taubitz T, Schraermeyer U. Different ef-fects of intravitreally injected ranibizumab and aflibercept on reti-nal and choroidal tissues of monkey eyes. Br J Ophthalmol. 2014; 98:813–25.

25. Kokame GT, Yeung L, Lai JC. Continuous anti-VEGF treatment with ranibizumab for polypoidal choroidal vasculopathy: 6-month results. Br J Ophthalmol. 2010; 94:297–301.

26. Inoue M, Arakawa A, Yamane S, Kadonosono K. Short-term effi-cacy of intravitreal aflibercept in treatment-naive patients with pol-ypoidal choroidal vasculopathy. Retina. 2014; 34:2178–84.

27. Sasahara M, Tsujikawa A, Musashi K. . Polypoidal choroidal vasculopathy with choroidal vascular hyperpermeability. Am J Ophthalmol. 2006; 142:601–7.

28. Chung SE, Kang SW, Lee JH, Kim YT. Choroidal thickness in pol-ypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 2011; 118:840–5.

29. Tan CS, Ouyang Y, Ruiz H, Sadda SR. Diurnal variation of choroi-dal thickness in normal, healthy subjects measured by spectral do-main optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53:261–6.

30. Wei WB, Xu L, Jonas JB. . Subfoveal choroidal thickness: the Beijing Eye Study. Ophthalmology. 2013; 120:175–80.

Figure 1.
Changes in best corrected visual acuity (BCVA), intraocular pressure (IOP), central macular thickness (CMT), subfoveal choroidal thickness (SFCT) after aflibercept injection. (A) BCVA was not changed significantly during the follow-up period com-pared with baseline. (B) IOP was decreased significantly 1, 2 months after aflibercept injection compared with baseline. (C) CMT was decreased significantly during the follow-up period compared with baseline. (D) SFCT was decreased significantly 1, 2 and 3 months after aflibercept injection compared with baseline. ETDRS = Early Treatment Diabetic Retinopathy Study. * Compared with baseline by repeated measure analysis of variance (ANOVA), p < 0.05.
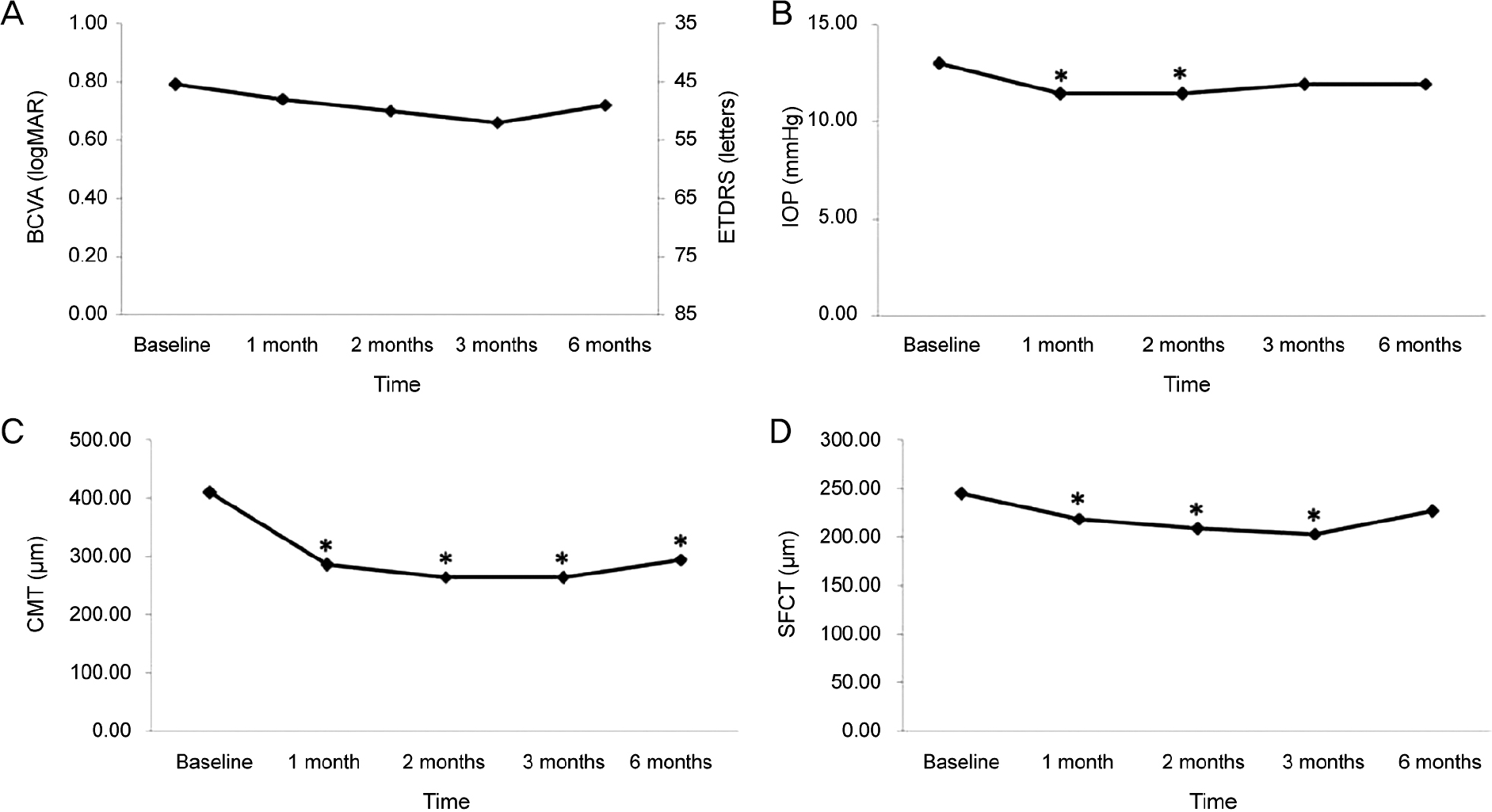
Figure 2.
Changes in best corrected visual acuity (BCVA), intraocular pressure (IOP), central macular thickness (CMT), subfoveal choroidal thickness (SFCT) after ranibizumab injection. (A) BCVA was not changed significantly during the follow-up period com-pared with baseline. (B) IOP was not changed significantly during the follow-up period compared with baseline. (C) CMT was de-creased significantly during the follow-up period compared with baseline. (D) SFCT was decreased significantly 1, 2 and 3 months after ranibizumab injection compared with baseline. ETDRS = Early Treatment Diabetic Retinopathy Study. * Compared with base-line by repeated measure analysis of variance (ANOVA), p < 0.05.
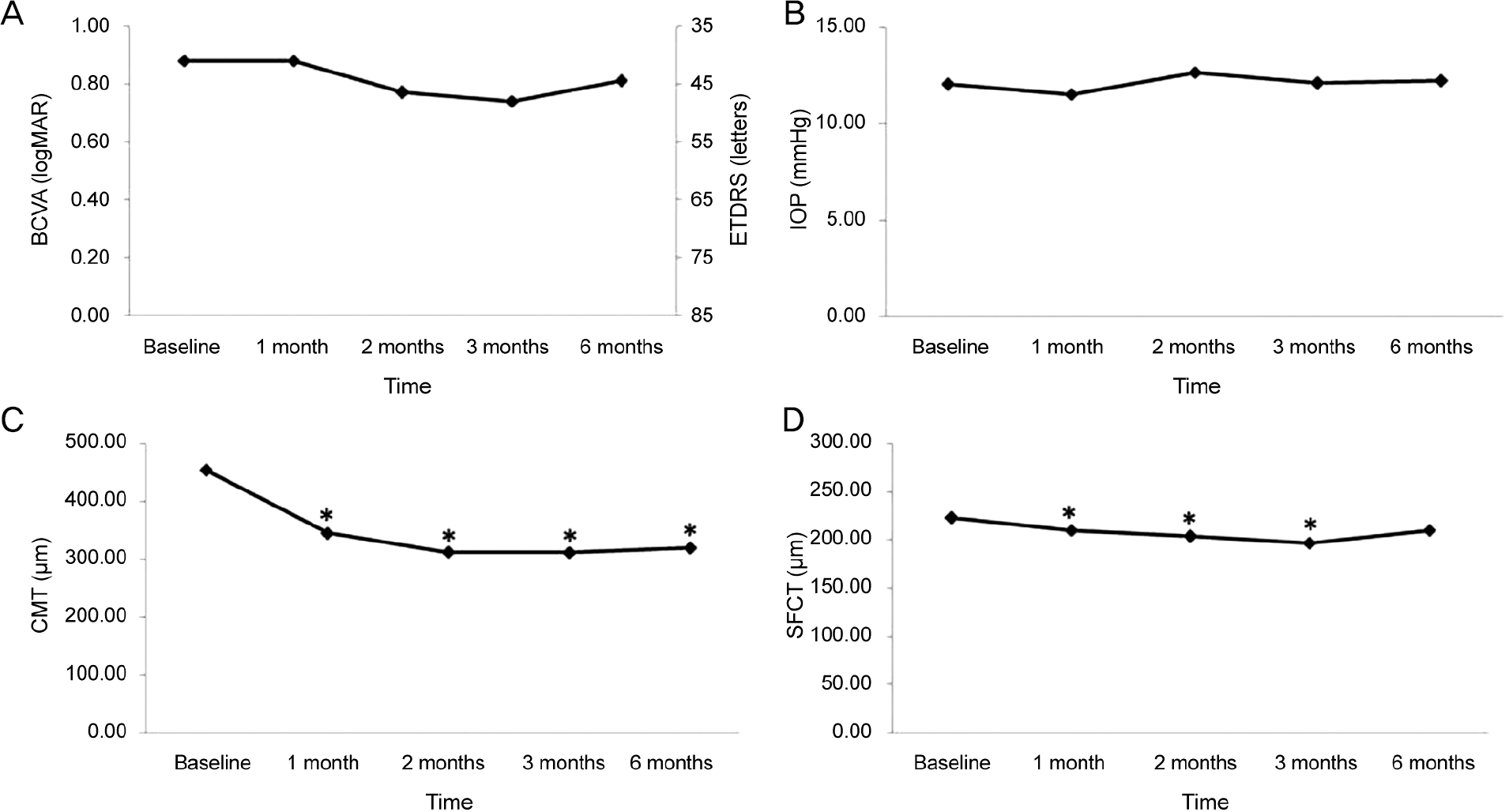
Figure 3.
Mean change in subfoveal choroidal thickness in aflibercept injection and ranibizumab injection. Subfoveal choroidal thickness was more decreased in the aflibercept in-jection group. Especially, there was a statistically significant difference between the two groups at 1, 2 and 3 months after injection. * Compared by Student’s t-test, p < 0.05.
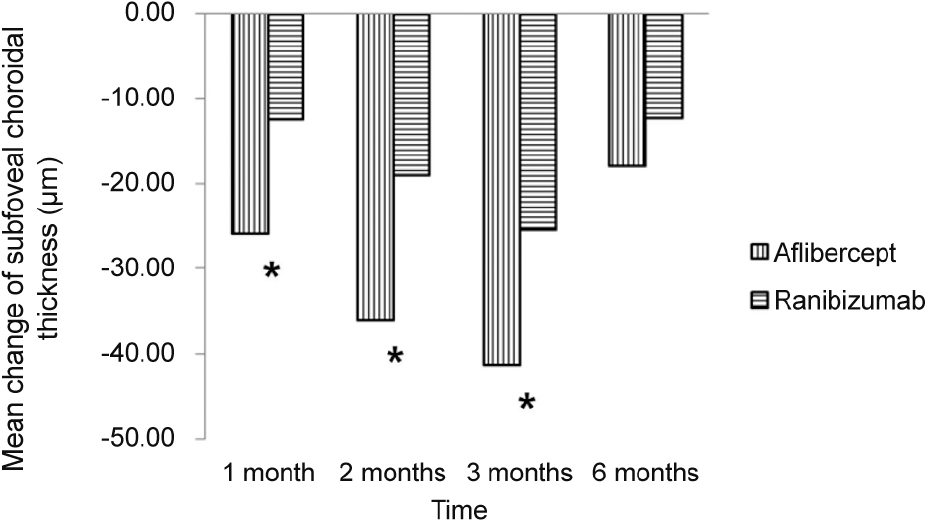
Figure 4.
Mean change in subfoveal choroidal thickness (SFCT) in typical wet age-related macular degeneration and polypoidal cho-roidal vasculopathy. (A) Mean change in SFCT in typical wet age-related macular degeneration. There was no significant difference between aflibercept and ranibizumab group ( p > 0.05). (B) Mean change SFCT in polypoidal choroidal vasculopathy. SFCT was more decreased in the aflibercept injection group at 1, 2 and 3 months after initial injection ( p < 0.05). * Compared by Student’s t-test, p < 0.05.
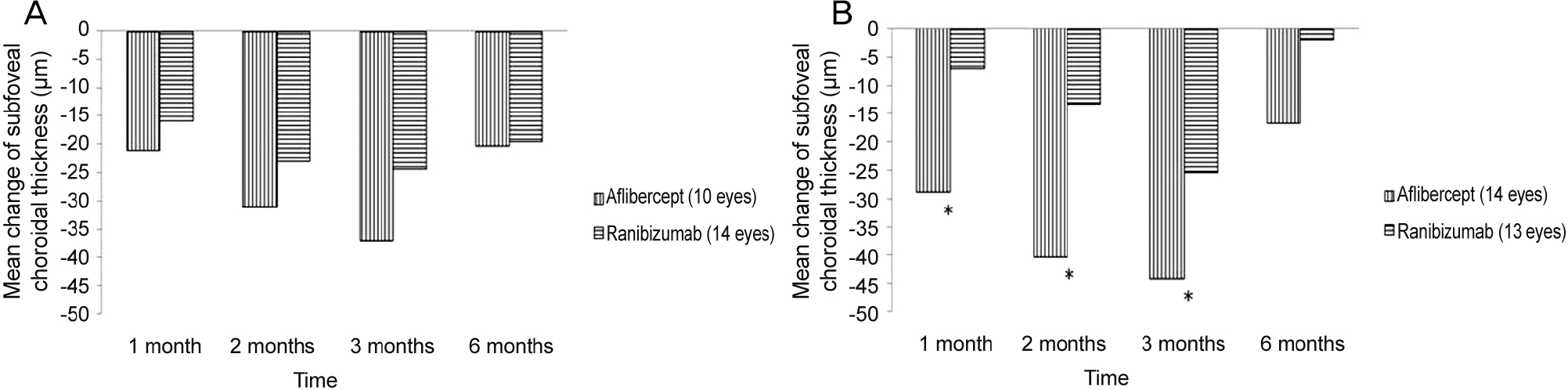
Figure 5.
Changes in best corrected visual acuity (BCVA), central macular thickness (CMT) and subfoveal choroidal thickness (SFCT) between only 3 monthly loading group and additional treatment after 3 loading dose group. (A) BCVA was not significantly different between the two groups after aflibercept injection. (B) BCVA was not significantly different between the two groups after ranibizumab injection. (C) CMT was not significantly different between the two groups after aflibercept injection. (D) CMT was not significantly different between the two groups after ranibizumab injection. (E) SFCT was not significantly different between the two groups after aflibercept injection. (F) SFCT was not significantly different between the two groups after ranibizumab injection. Compared by Student’s t-test. ETDRS = Early Treatment Diabetic Retinopathy Study.
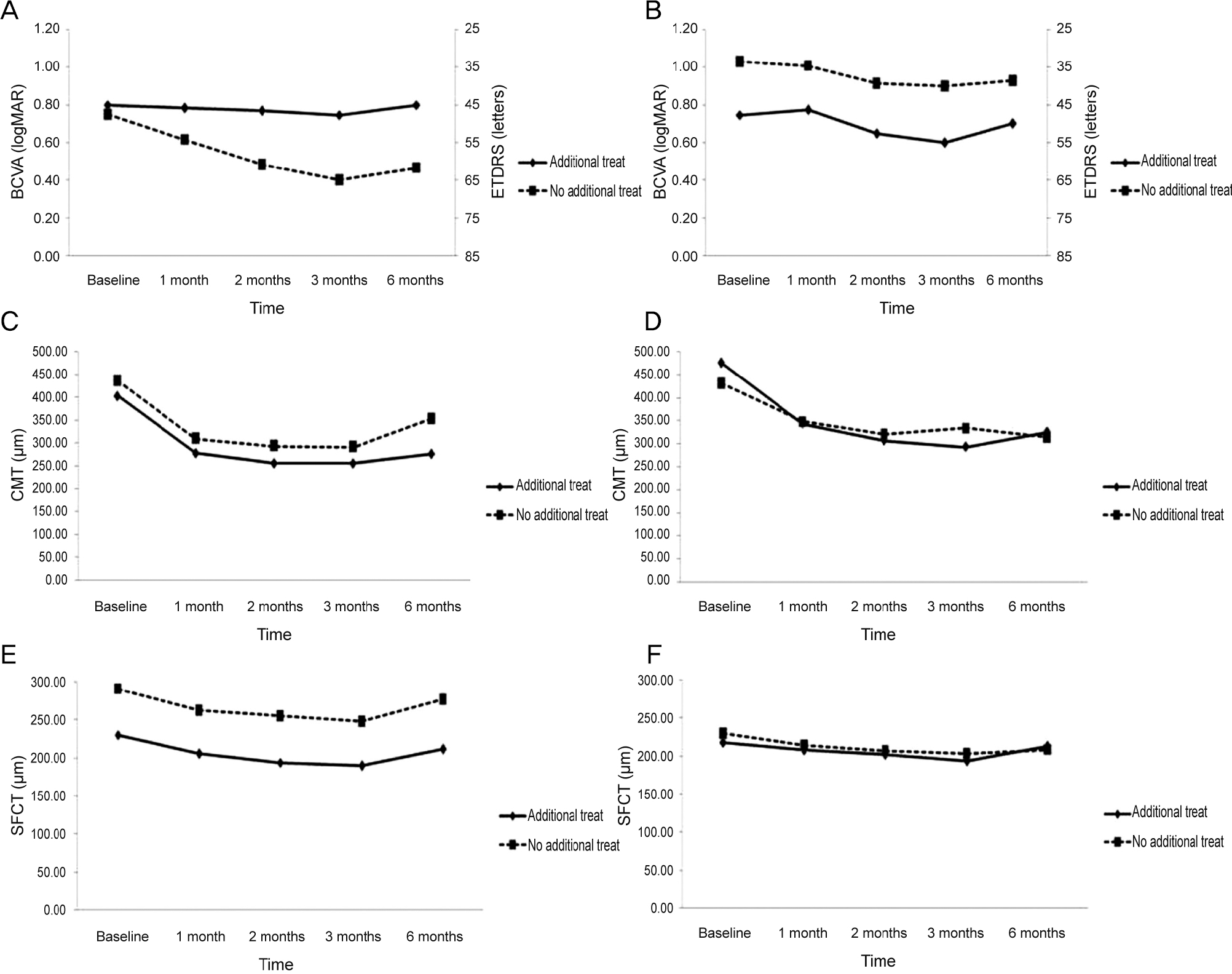
Table 1.
Demographics of the study groups
| Characteristics | Aflibercept | Ranibizumab | p-value |
|---|---|---|---|
| Number of eyes Sex (male:female) | 2521:4 | 28 21:7 | 0.420* |
| Laterality (OD:OS) | 9:16 | 19:9 | 0.020* |
| Mean age (years) | 68.88 ± 7.68 | 71.50 ± 7.41 | 0.212† |
| Diabetes mellitus (patients) | 3 | 2 | 0.883* |
| Hypertension (patients) | 5 | 5 | 0.664* |
| Best corrected visual acuity (logMAR) | 0.79 ± 0.36 | 0.88 ± 0.51 | 0.423‡ |
| Intraocular pressure (mmHg) | 13.00 ± 3.43 | 12.04 ± 2.55 | 0.494‡ |
| Spherical equivalent (diopter) | 0.63 ± 0.91 | 0.18 ± 1.13 | 0.126† |
| Macular center thickness (μ m) | 410.56 ± 118.13 | 454.68 ± 113.98 | 0.153† |
| Subfoveal choroidal thickness (μ m) | 244.94 ± 103.77 | 222.68 ± 102.04 | 0.473† |
| Number of injections | 3.24 ± 0.44 | 3.75 ± 0.93 | 0.037‡ |
| Age-related macular degeneration type | |||
| Typical wet age-related macular degeneration (eyes) | 10 | 14 | |
| Polypoidal choroidal vasculopathy (eyes) | 14 | 13 | |
| Retinal angiomatous proliferation (eyes) | 1 | 1 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download