Abstract
Purpose
Methods
Results
Conclusions
Figures and Tables
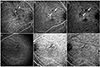 | Figure 1Morphological classification of polypoidal choroidal vasculopathy (PCV) based on indocyanine green angiography (ICGA). ICGA of a patient with type 1 polypoidal choroidal neovascularization (CNV), a feeder vessel (white arrowheads) is visible at 20s (A). At 59s (B), a drainage vessel is observed (white arrows). In the subtraction (A from B) image (C), the feeder vessel was not showed but the drainage vessel (white arrows) was visible. ICGA of a patient with type 2 polypoidal CNV, the small weak branch vascular network (BVN) (black arrow) and choroidal vessels are enhanced at 23s (D). At 58s (E), a polyp and small BVN (black arrow) are more prominent. In the subtraction (D from E) image (F), the small BVN (black arrow) was still visible, which indicates slowing of the vessel filling. |
 | Figure 2Comparison of the complete polyp regression between the aflibercept-and ranibizumab-treated groups. The aflibercept-treated group showed a better polyp regression than the ranibizumab-treated group in patients with type 1 polypoidal choroidal vasculopathy (PCV) (p = 0.020), while patients with type 2 PCV showed no significant difference between the two groups (p = 0.436). Patients with type 1 PCV showed a significantly higher regression percentage than patients with type 2 PCV in the aflibercept-treated group (p = 0.020), while the ranibizumab-treated group showed no significant difference between PCV types (p = 0.370). *p < 0.05, Fisher's exact test. |
 | Figure 3Outcomes of the 6-month treatment effects and comparisons between anti-vascular endothelial growth factor (anti-VEGF) agents. The aflibercept-treated group showed a better response than the ranibizumab-treated group (A, D, G, J). The anatomical changes were greater in patients treated with aflibercept than ranibizumab, particularly in patients with type 1 polypoidal choroidal vasculopathy (PCV) (B, E, H, K). In patients with type 2 PCV (C, F, I, L), there was no significant visual and anatomical difference between the anti-VEGF agents. BCVA = best-corrected visual acuity; PED = pigment epithelial detachment; CMT = central macular thickness; CT = choroidal thickness. *Parameters significantly changed from baseline (p < 0.05, Mann-Whitney U-test); †Changes in treatment effects that were significantly different between the anti-VEGF agents (p < 0.05, Mann-Whitney U test). |
Table 1
Baseline characteristics of patients with type 1 PCV and type 2 PCV

PCV = polypoidal choroidal vasculopathy; BCVA = best-corrected visual acuity; logMAR = logarithm of the minimum angle of resolution; GLD = greatest linear dimension; BVN = branch vascular network; PED = pigment epithelial detachment; CMT = central macular thickness; CT = choroidal thickness.
*Data are presented as mean ± SD; †p-value using the Mann-Whitney test; ‡p-value using Fisher's exact test; §Comparison between Aflibercept group and Ranivizumab group; ΠComparison between Type 1 PCV and Type 2 PCV.
Table 2
A comparison of anatomical and functional changes between aflivercept- and ranibizumab-treated groups according to the angiographic of PCV

PCV = polypoidal choroidal vasculopathy; BCVA = best-corrected visual acuity; logMAR = logarithm of the minimum angle of resolution; PED = pigment epithelial detachment; CMT = central macular thickness; CT = choroidal thickness.
*Data are presented as mean ± SD; †p-value using Fisher's exact test; ‡p-value using the Mann-Whitney test; §Comparison between Aflibercept group and Ranivizumab group; ΠComparison between Type 1 PCV and Type 2 PCV.
Table 3
A comparison of the effects of intravitreal aflibercept and ranibizumab on anatomical and functional parameters in each patient group with type 1 and type 2 PCV

PCV = polypoidal choroidal vasculopathy; BCVA = best-corrected visual acuity; logMAR = logarithm of the minimum angle of resolution; PED = pigment epithelial detachment; CMT = central macular thickness; CT = choroidal thickness.
*Data are presented as mean ± SD; †p-value using the Wilcoxon signed-rank test; ‡Comparison between before and 3 month after intravitreal injection of anti-vascular endothelial growth factor (anti-VEGF); §Comparison between before and 6 month after intravitreal injection of anti-VEGF.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download