Abstract
Purpose
To compare the effects of anti-inflammatory agents, specifically bromfenac, loteprednol, and prednisolone, on the permeability of cultured human trabecular meshwork cell (HTMC) monolayers.
Methods
HTMCs were cultured until confluency in the inner chamber of Transwell, then exposed to 1/1,000 or 1/500 diluted commercial 0.1% bromfenac, 0.5% loteprednol, and 1% prednisolone for 24 hours. The permeabilities of carboxyfluorescein through the HTMC monolayer were measured with a spectrofluorometer after 2 hours in the outer chamber. Cellular viabilities were assessed with an 3-[4,5– dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay.
Results
Bromfenac and loteprednol diluted at 1/1,000 or 1/500 did not significantly affect the cellular survival (p > 0.05). Bromfenac did not affect the permeability via the HTMC monolayer (p > 0.05) and loteprednol decreased the permeability (p < 0.05). In addition, 1/2,000 prednisolone also decreased the permeability (p < 0.05).
Go to : 
References
2. Noble S, Goa KL. Loteprednol etabonate: clinical potential in the management of ocular inflammation. BioDrugs. 1998; 10:329–39.
3. Pavesio CE, DeCory HH. Treatment of ocular inflammatory abdominal with loteprednol etabonate. Br J Ophthalmol. 2008; 92:455–9.
4. Amon M, Busin M. Loteprednol etabonate ophthalmic suspension 0.5 %: efficacy and safety for postoperative anti-inflammatory use. Int Ophthalmol. 2012; 32:507–17.
5. Bodor N, Bodor N, Wu WM. A comparison of intraocular pressure elevating activity of loteprednol etabonate and dexamethasone in rabbits. Curr Eye Res. 1992; 11:525–30.

6. Rajpal RK, Digby D, D'Aversa G, et al. Intraocular pressure abdominal with loteprednol etabonate: a retrospective chart review. J Ocul Pharmacol Ther. 2011; 27:305–8.
7. Lane SS, Holland EJ. Loteprednol etabonate 0.5% versus abdominal acetate 1.0% for the treatment of inflammation after abdominal surgery. J Cataract Refract Surg. 2013; 39:168–73.
8. Controlled evaluation of loteprednol etabonate and prednisolone acetate in the treatment of acute anterior uveitis. Loteprednol Etabonate US Uveitis Study Group. Am J Ophthalmol. 1999; 127:537–44.
9. Koay P. The emerging roles of topical non-steroidal antiabdominal agents in ophthalmology. Br J Ophthalmol. 1996; 80:480–5.
10. Nichols J, Snyder RW. Topical nonsteroidal anti-inflammatory agents in ophthalmology. Curr Opin Ophthalmol. 1998; 9:40–4.

11. Gaynes BI, Fiscella R. Topical nonsteroidal anti-inflammatory drugs for ophthalmic use: a safety review. Drug Saf. 2002; 25:233–50.
12. Walters T, Raizman M, Ernest P, et al. In vivo pharmacokinetics and in vitro pharmacodynamics of nepafenac, amfenac, ketorolac, and bromfenac. J Cataract Refract Surg. 2007; 33:1539–45.

13. Stewart RH, Grillone LR, Shiffman ML, et al. The systemic safety of bromfenac ophthalmic solution 0.09%. J Ocul Pharmacol Ther. 2007; 23:601–12.

14. Walters TR, Goldberg DF, Peace JH, et al. Bromfenac ophthalmic solution 0.07% dosed once daily for cataract surgery: results of 2 randomized controlled trials. Ophthalmology. 2014; 121:25–33.
15. Mosmann T. Rapid colorimetric assay for cellular growth and abdominal: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.
16. Fremoser FM, Jakob CA, Aebi M, Tuor U. The MTT [3-(4,5-dime-thylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay is a fast and reliable method for colorimetric determination of fungal cell densities. Appl Environ Microbiol. 1999; 65:3727–9.

17. Grimes PA, Stone RA, Laties AM, Li W. Carboxyfluorescein. A probe of the blood-ocular barriers with lower membrane abdominal than fluorescein. Arch Ophthalmol. 1982; 100:635–9.
18. Araie M. Carboxyfluorescein. A dye for evaluating the corneal abdominal barrier function in vivo. Exp Eye Res. 1986; 42:141–50.
19. Grimes PA. Carboxyfluorescein transfer across the blood-retinal barrier evaluated by quantitative fluorescence microscopy: abdominal with fluorescein. Exp Eye Res. 1988; 46:769–83.
20. Nakagawa S, Usui T, Yokoo S, et al. Toxicity evaluation of abdominal drugs using stratified human cultivated corneal abdominal sheets. Invest Ophthalmol Vis Sci. 2012; 53:5154–60.
21. Lei Y, Stamer WD, Wu J, Sun X. Oxidative stress impact on barrier function of porcine angular aqueous plexus cell monolayers. Invest Ophthalmol Vis Sci. 2013; 54:4827–35.

22. Raghunathan VK, Morgan JT, Park SA, et al. Dexamethasone stif-fens trabecular meshwork, trabecular meshwork cells, and matrix. Invest Ophthalmol Vis Sci. 2015; 56:4447–59.

23. Clark AF. Basic sciences in clinical glaucoma: steroids, ocular abdominal, and glaucoma. J Glaucoma. 1995; 4:354–69.
24. Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics. I. The effect of dexamethasone in the normal eye. Arch Ophthalmol. 1963; 70:482–91.
25. Armaly MF: Effect of corticosteroids on intraocular pressure and fluid dynamics. II. The effect of dexamethasone in the abdominal eye. Arch Ophthalmol. 1963; 70:492–9.
26. Becker B. Intraocular pressure response to topical corticosteroids. Invest Ophthalmol. 1965; 4:198–205.
27. Cantrill HL, Palmberg PF, Zink HA, et al. Comparison of in vitro potency of corticosteroids with ability to raise intraocular pressure. Am J Ophthalmol. 1975; 79:1012–7.

28. Wilhelmi E. Experimental and clinical investigation of a non-hor-monal anti-inflammatory eye ointment. Ophthalmic Res. 1973; 5:253–89.

29. Strelow SA, Sherwood MB, Broncato LJ, et al. The effect of abdominal sodium ophthalmic solution on intraocular pressure abdominal cataract extraction. Ophthalmic Surg. 1992; 23:170–5.
30. Gieser DK, Hodapp E, Goldberg I, et al. Flubiprofen and abdominal pressure. Ann Ophthalmol. 1981; 13:831–3.
31. Podos SM, Becker B, Morton WR. High myopia and primary open-angle glaucoma. Am J Ophthalmol. 1966; 62:1038–43.

32. Becker B. Diabetes mellitus and primary open-angle glaucoma. The XXVII Edward Jackson Memorial Lecture. Am J Ophthalmol. 1971; 71(1 Pt 1):1–16.
33. Williams DE, Nguyen KD, Shapourifar-Tehrani S, et al. Effects of timolol, betaxolol, and levobunolol on human tenon's fibroblasts in tissue culture. Invest Ophthalmol Vis Sci. 1992; 33:2233–41.
Go to : 
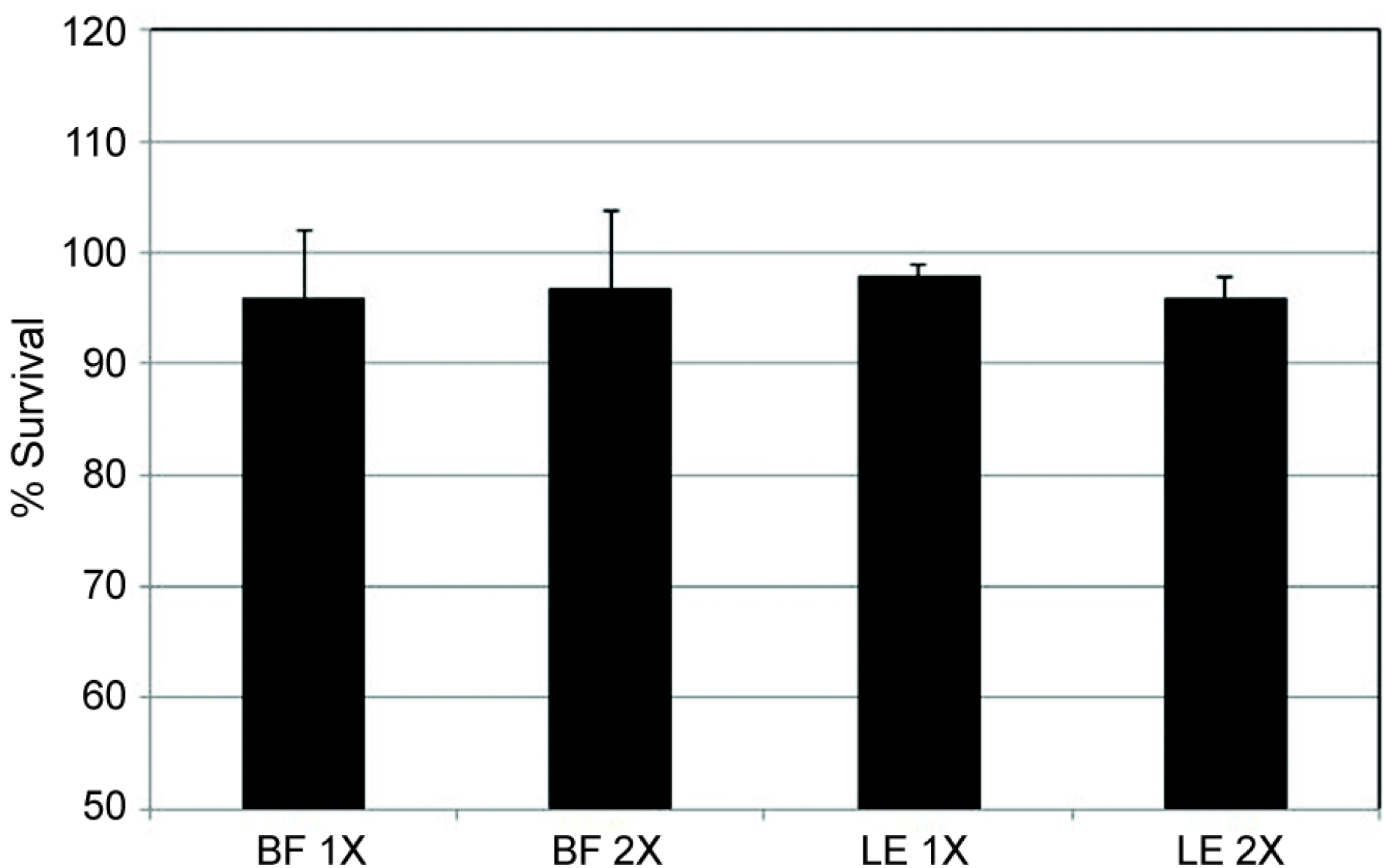 | Figure 1.Effect of bromfenac sodium (BF) and loteprednol etabonate (LE) on the survival of human trabecular meshwork cells in tissue culture. Both bromfenac sodium and loteprednol did not affect the survival of trabecular meshwork cells compared to non-exposed controls (X= 1/1,000 dilution). |
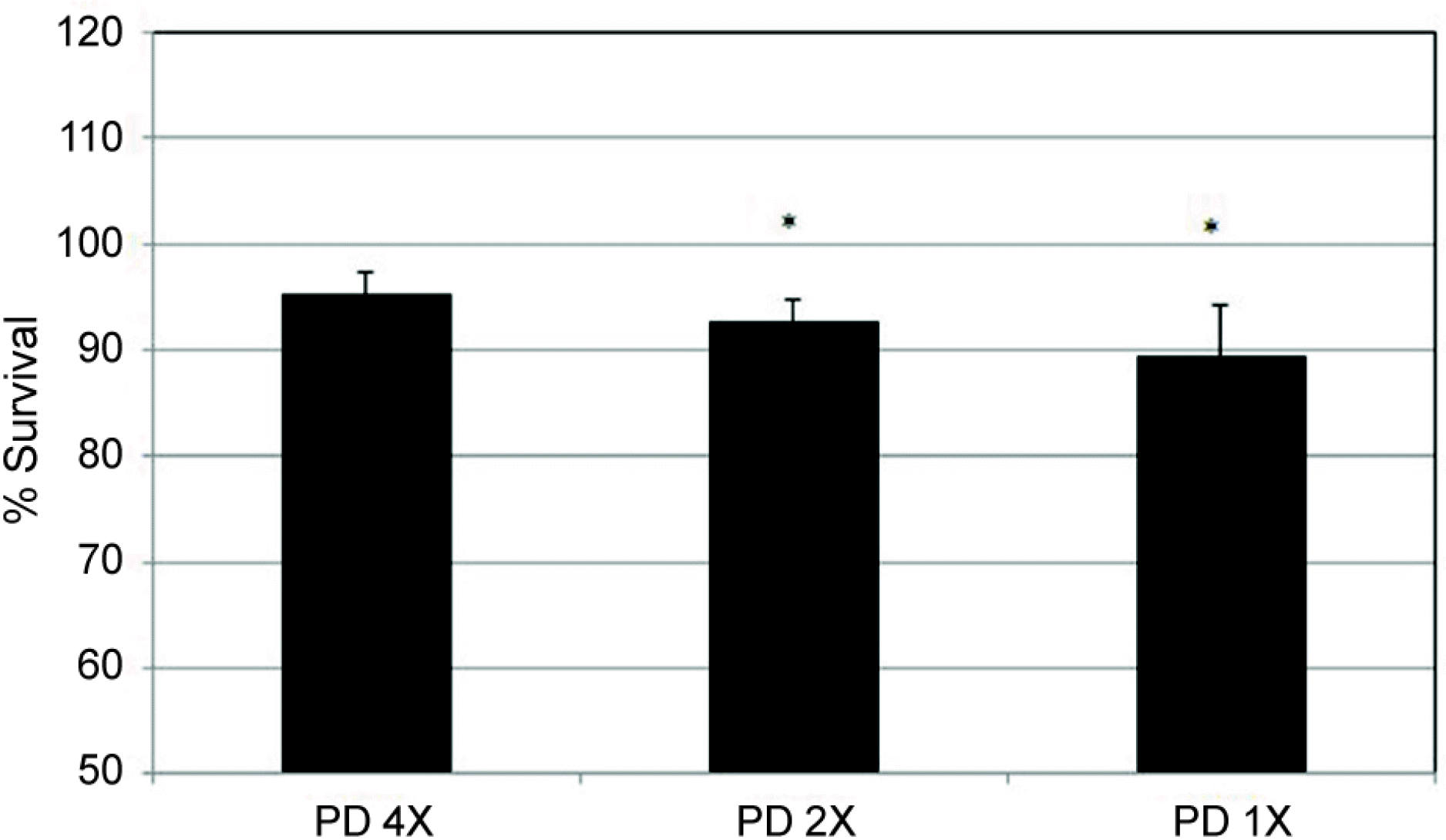 | Figure 2.Effect of prednisolone acetate (PD) on the survival of human trabecular meshwork cells in tissue culture. Exposure to 1/1,000 or 1/500 diluted prednisolone decreased the survival of trabecular meshwork cells compared to non-ex-posed controls (* p < 0.05) (X = 1/1,000 dilution). |
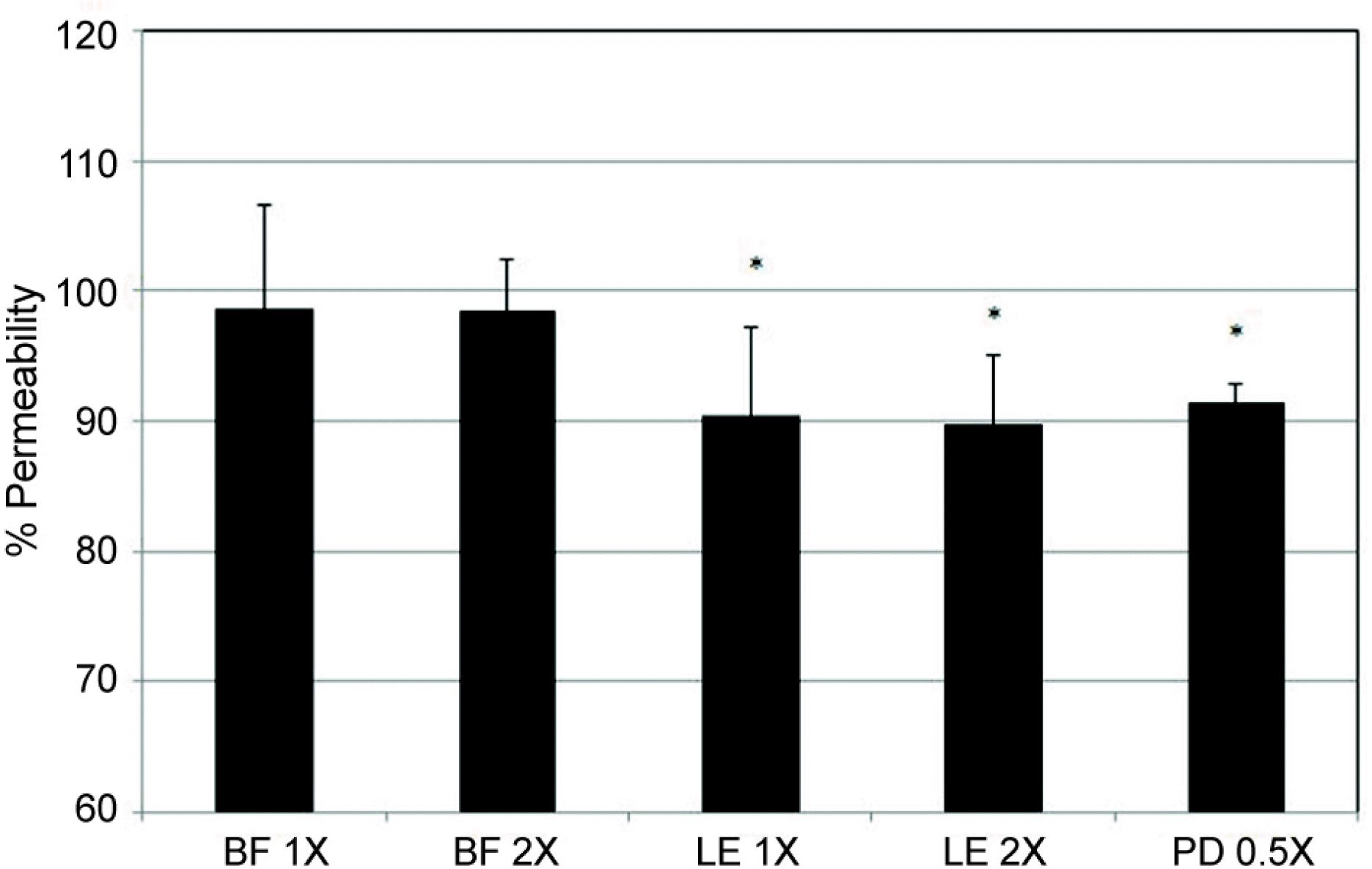 | Figure 3.Effects of bromfenac sodium (BF), loteprednol ta-bonate (LE), or prednisolone acetate (PD) on the permeability of carboxyfluorescin through the trabecular meshwork cell monolayer. Both loteprednol and prednisolone decreased the permeabilty of carboxyfluorescein significantly compared control using phosphated buffered saline (PBS) (* p < 0.05). Carboxyfluorescein intensity of outer chamber normalized to the mean value obtained using PBS (permeability 100%) (X= 1/1,000 dilution). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download