Abstract
Purpose
To analyze the anatomical characteristics on spectraldomain optical coherence tomography (SD-OCT) of patients who are legally blind (less than 20/1,000) due to end-stage exudative age-related macular degeneration (AMD) that does not require intravitreal anti-vascular endothelial growth factor (anti-VEGF) injection.
Methods
After anti-VEGF injections (active group), 120 eyes of 103 exudative AMD patients experienced visual acuity improved by at least 2 lines or improvement on SD-OCT. In addition, 55 eyes of 54 end-stage exudative AMD patients who did not respond to treatment or who were legally blind due to foveal scar at the first visit (end-stage group) were evaluated retrospectively. Changes in retinal structures of the 2 groups were analyzed by SD-OCT at the last visit.
Results
The mean age of the end-stage group was about 5 years older than the active group. During the follow-up period, subretinal hemorrhage, intraretinal hemorrhage and retinal pigment epithelium tear occurred more frequently in the end-stage group than in the active group (p < 0.05). Intra-retinal fluids and subretinal fluids were more frequently administered in the active group than in the end-stage group, and thick subretinal hyper-reflective materials (SRHRM), fibrovascular pigment epithelial detachment (PED) and extensive inner segment/outer segment (IS/OS) line disruption were observed in all eyes of the end-stage group. The size and thickness of PED, foveal thickness and SRHRM thickness were significantly larger in the end-stage group than in the active group (p < 0.05). Disciform retinal scars were eventually formed in most of the end-stage group.
Conclusions
In end-stage exudative AMD, the presence of retinal hemorrhage and retinal pigment epithelium tear during fol-low-up, or the findings of thick SRHRM, fibrovascular PED, and extensive IS/OS line disruption on SD-OCT suggest weak expected effect of intravitreal anti-VEGF injection, which can act as a reference for determining the timing of treatment termination.
Go to : 
References
1. Wong TY, Chakravarthy U, Klein R, et al. The natural history and prognosis of neovascular age-related macular degeneration: a abdominalic review of the literature and meta-analysis. Ophthalmology. 2008; 115:116–26.
2. Sunness JS, Gonzalez-Baron J, Applegate CA, et al. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology. 1999; 106:1768–79.

3. Pauleikhoff D. Neovascular age-related macular degeneration: abdominal history and treatment outcomes. Retina. 2005; 25:1065–84.
4. Bressler SB. Introduction: understanding the role of angiogenesis and anti-angiogenic agents in age-related macular degeneration. Ophthalmology. 2009; 116(10 Suppl):S1–7.

5. Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003; 22:1–29.

6. Green WR, Enger C. Age-related macular degeneration abdominal studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993; 100:1519–35.
7. Wilgus TA, Ferreira AM, Oberyszyn TM, et al. Regulation of scar formation by vascular endothelial growth factor. Lab Invest. 2000; 8:579–90.

8. Michels S, Rosenfeld PJ, Puliafito CA, et al. Systemic abdominal (Avastin) therapy for neovascular age-related macular degeneration twelve-week results of an uncontrolled open-label clinical study. Ophthalmology. 2005; 112:1035–47.
9. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–31.

10. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus abdominal for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–44.
11. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–31.

12. Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal abdominal (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007; 143:566–83.
13. Gupta OP, Shienbaum G, Patel AH, et al. A treat and extend abdominal using ranibizumab for neovascular age-related macular abdominal clinical and economic impact. Ophthalmology. 2010; 117:2134–40.
14. Cohen SY, Oubraham H, Uzzan J, et al. Causes of unsuccessful abdominal treatment in exudative age-related macular abdominal in clinical settings. Retina. 2012; 32:1480–5.
15. Daniel E, Toth CA, Grunwald JE, et al. Risk of scar in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014; 121:656–66.

16. Rahimy E, Freund KB, Larsen M, et al. Multilayered pigment abdominal detachment in neovascular age-related macular degeneration. Retina. 2014; 34:1289–95.
17. Cho SW, Bae JH, Song SJ. Anatomical non-responder to intravitreal bevacizumab for neovascular age-related macular degeneration. J Korean Ophthalmol Soc. 2010; 51:1464–70.

18. Shin JY, Woo SJ, Ahn J, Park KH. Anti-VEGF-refractory abdominal age-related macular degeneration: differential response abdominal to features on optical coherence tomography. J Korean Ophthalmol. 2013; 27:425–32.
19. Varshney N, Jain A, Chan V, et al. Anti-VEGF response in macular hemorrhage and incidence of retinal pigment epithelial tears. Can J Ophthamol. 2013; 48:210–5.

20. Empeslidis T, Vardarinos A, Konidaris V, et al. Incidence of retinal pigment epithelial tears and associated risk factors after treatment of age-related macular degeneration with intravitreal Anti-VEGF Injections. Open Ophthalmol J. 2014; 8:101–4.

21. Gutfleisch M, Heimes B, Schumacher M, et al. abdominal visual outcome of pigment epithelial tears in association with anti-VEGF therapy of pigment epithelial detachment in AMD. Eye (Lond). 2011; 25:1181–6.
22. Durkin SR, Farmer LD, Kulasekara S, Gilhotra J. Change in vision after retinal pigment epithelium tear following the use of an-ti-VEGF therapy for age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2016; 254:1–6.

23. Jaffe GJ, Martin DF, Toth CA, et al. Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Macular morphology and visual acuity in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2013; 120:1860–70.
24. Golbaz I, Ahlers C, Stock G, et al. Quantification of the therapeutic response of intraretinal, subretinal, and subpigment epithelial com-partments in exudative AMD during anti-VEGF therapy. Invest Ophthalmol Vis Sci. 2011; 52:1599–605.

25. Simader C, Ritter M, Bolz M, et al. Morphologic parameters relevant for visual outcome during anti-angiogenic therapy of neovascular age-related macular degeneration. Ophthalmology. 2014; 121:1237–45.

26. Ores R, Puche N, Querques G, et al. Gray abdominal subretinal exudative lesions in exudative age-related macular degeneration. Am J Ophthalmol. 2014; 158:354–61.
27. Shah VP, Shah SA, Mrejen S, Freund KB. Subretinal abdominal exudation associated with neovascular age-related abdominal degeneration. Retina. 2014; 34:1281–8.
Go to : 
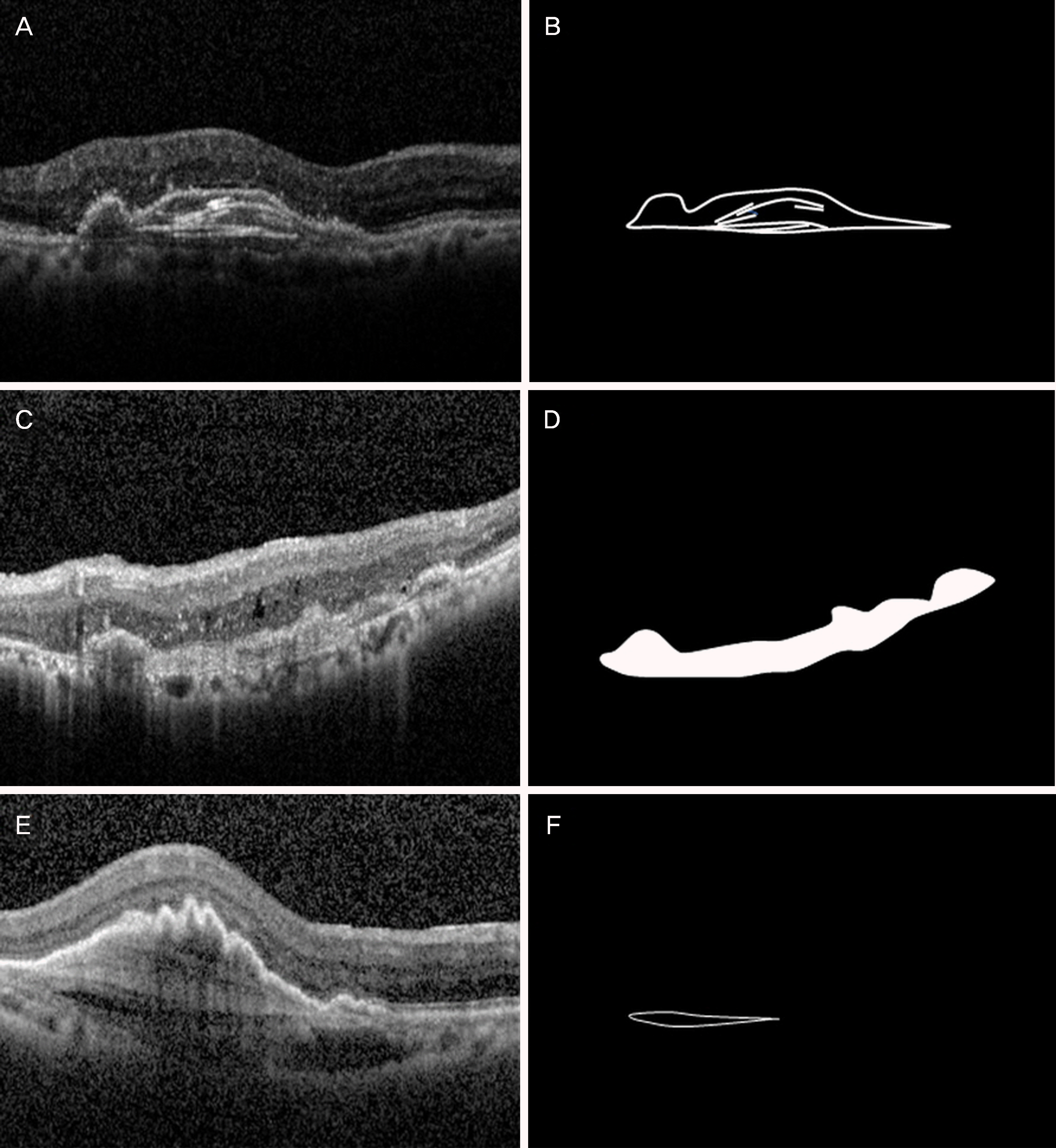 | Figure 1.Representative images of morphologic features of spectraldomain optical coherence tomography (SD-OCT) related to age-related macular degeneration. SD-OCT image (A) and corresponding schematic image (B) show multilayered fibrovascular pigment epithelial detachment (f-PED): a fusiform, or spindle-shaped, complex of organized layers of homogenous hyperreflective bands. SD-OCT image (C) and corresponding schematic image (D) demonstrate irregular f-PED with heterogenous, hyper-reflective, dilated, irregular vascular networks. SD-OCT image (E) and corresponding schematic image (F) depict hyporeflective prechoroidal cleft separating the neovascular tissue complex from the underlying choroid and Bruch's membrane. |
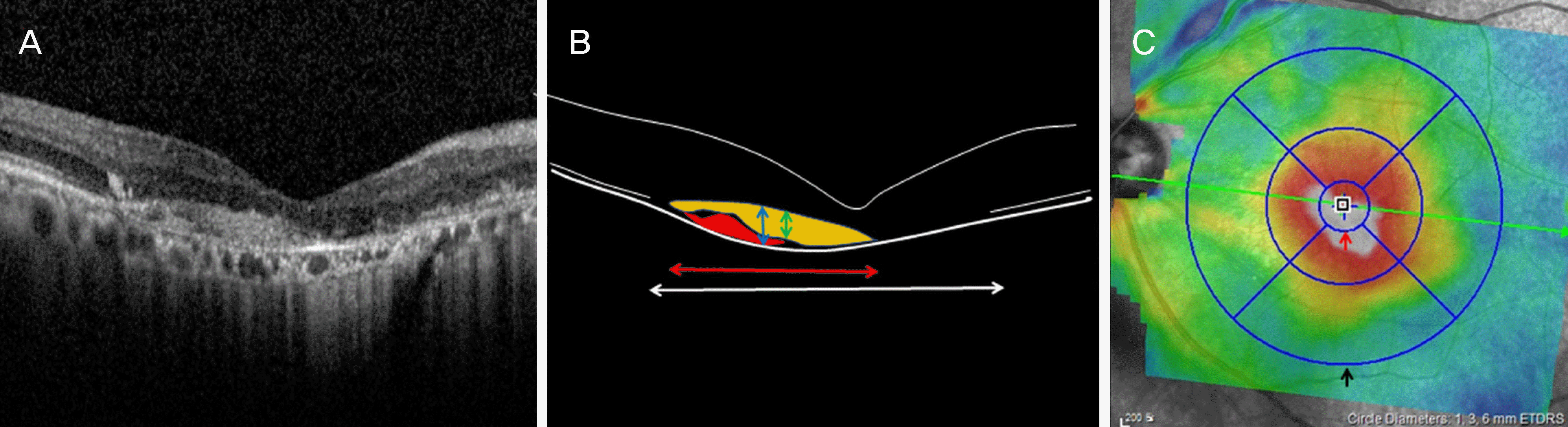 | Figure 2.Measurement of features of spectraldomain optical coherence tomography related to age-related macular degeneration using Heidelberg engineering software. Representative image of end-stage age-related macular degeneration (A) and corresponding schematic image (B) show thickness (B, blue left right arrow) and size (red left right arrow) of pigment epithelial detachment (PED), thickness of subretinal hyperreflective materials (B, green left right arrow) and size of defect of photoreceptor layer (B, white left right arrow). PED contains choroidal neovascularization (B, red colored lesion) and subretinal hyperreflective materials (B, yellow colored lesion). Macular 1 mm circle thickness and macular volume is measured by using macular 1 mm circle (C, red arrow), macular 6 mm circle (C, black arrow) of Color-coded thickness map. |
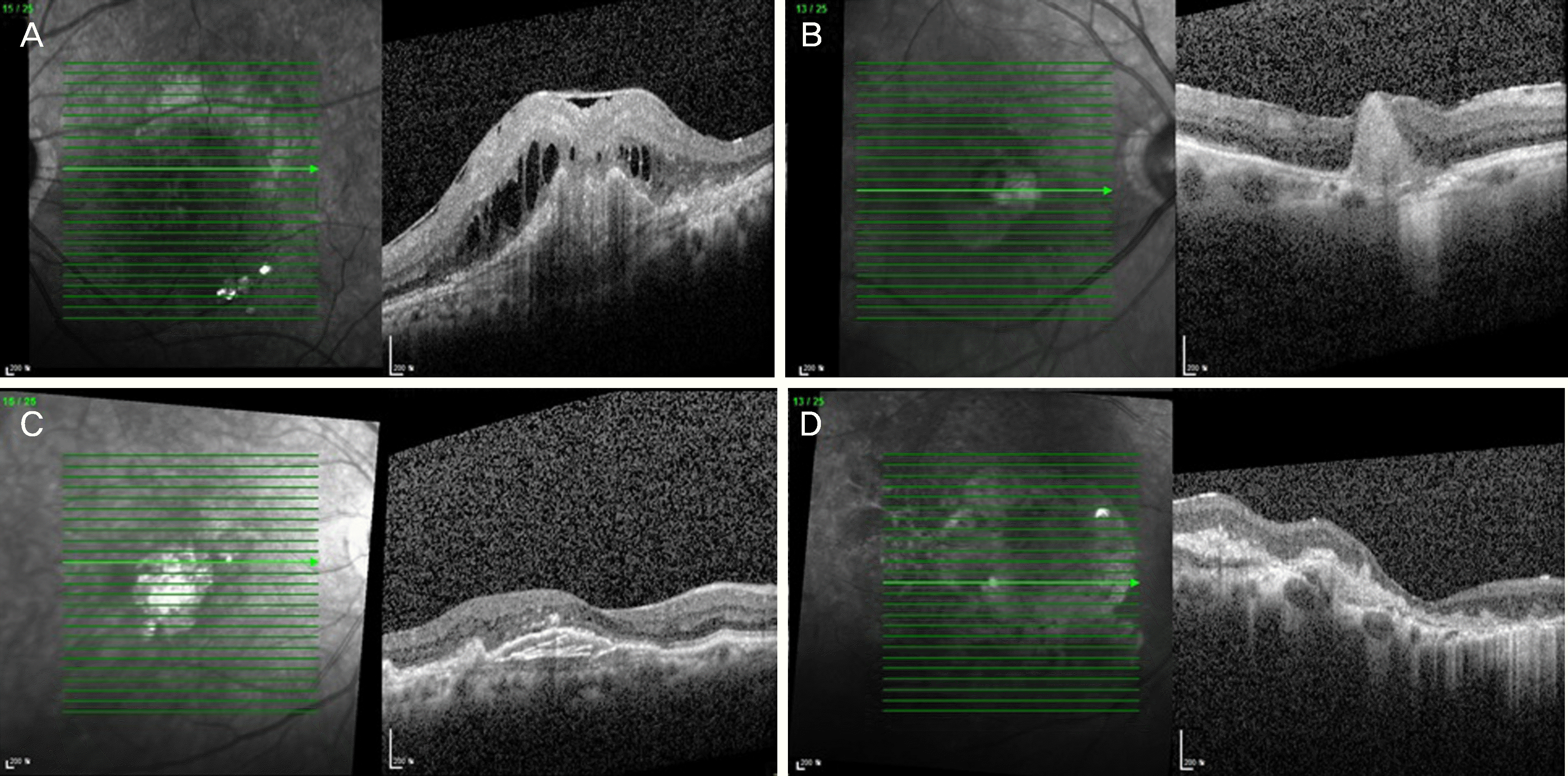 | Figure 3.Four types of end-stage age-related macular degeneration. Spectral-domain optical coherence tomography (SD-OCT) images. (A) Shows disciform scar with intra-retinal cystoid fluid. SD-OCT image (B) demonstrates disciform scar with destruction of retinal layers. SD-OCT image (C) shows multilayered pigment epithelial detachment (PED) with broad inner segment/outer segment line disruption. SD-OCT image (D) demonstrates thin irregular fibrovascular PED with atrophy of inner retinal layer and inner segment/outer segment line disruptions. |
Table 1.
Demographic data of the patients
| Characteristics | Active (n = 120) | End-stage (n = 55) | p-value* |
|---|---|---|---|
| Age (years) | 69.82 ± 8.24 | 74.75 ± 6.39 | 0.037 |
| Sex (male:female) | 50:70 | 19:36 | 0.408 |
| Mean BCVA (logMAR) | 0.53 ± 0.51 | 2.02 ± 0.18 | <0.001 |
| Lens state (n, %) | |||
| Phakia | 79 (65.83) | 35 (63.64) | 0.864 |
| Pseudophakia | 41 (34.17) | 19 (34.54) | 0.546 |
| Aphakia | 0 (0) | 1 (1.82) | 0.314 |
| Retinal events during follow up (n, %) | |||
| Subretinal hemorrhage | 13 (10.74) | 19 (34.55) | <0.001 |
| Intra-retinal hemorrhage | 9 (7.44) | 16 (29.10) | <0.001 |
| RPE tear | 8 (6.61) | 37 (67.73) | <0.001 |
| Received treatment during follow up (n, %) | |||
| Naïve eyes | 0 (0) | 4 (7.27) | 0.009 |
| Intravitreal Anti-VEGF injection | 94 (78.33) | 32 (58.19) | 0.010 |
| PDT | 0 (0) | 1 (1.81) | 0.314 |
| Intravitreal Anti-VEGF injection + PDT | 26 (21.67) | 18 (32.73) | 0.135 |
Table 2.
Comparison of OCT findings of active AMD and end-stage AMD
| Active | End-stage | p-value* | |
|---|---|---|---|
| Subretinal fluid (n, %) | 67 (55.83) | 17 (30.91) | 0.003 |
| Intraretinal cystoid fluid (n, %) | 93 (77.50) | 34 (61.82) | 0.044 |
| Subretinal hyperreflective material (n, %) | 42 (35.00) | 55 (100.0) | <0.001 |
| Prechoroidal cleft (n, %) | 5 (4.16) | 3 (5.45) | 0.708 |
| Atrophic scar (n, %) | 2 (1.67) | 4 (7.27) | 0.079 |
| Disciform scar (n, %) | 11 (9.16) | 49 (89.09) | <0.001 |
| PED (n, %) | 108 (90.00) | 55 (100) | 0.019 |
| Serous PED | 58 (48.33) | 0 (0) | <0.001 |
| Drusenoid PED | 12 (10.00) | 0 (0) | 0.019 |
| Hemorrhagic PED | 8 (6.67) | 0 (0) | 0.057 |
| Irregular f-PED | 28 (23.33) | 51 (92.72) | <0.001 |
| Multilayered f-PED | 2 (1.67) | 4 (7.27) | 0.058 |
Table 3.
Statistical analysis of anatomical characteristics of active AMD and end-stage AMD
| Active | End-stage | p-value* | |
|---|---|---|---|
| PED size (μ m) | 2,123.37 ± 1,184.55 | 3,747.83 ± 1,460.09 | 0.003 |
| PED thickness (μ m) | 179.15 ± 152.27 | 301.27 ± 160.62 | 0.001 |
| IS/OS defect size (μ m) | 593.21 ± 1,647.36 | 5,078.27 ± 1,386.17 | 0.001 |
| FT (μ m) | 237.28 ± 57.05 | 375.80 ± 39.68 | 0.002 |
| Macular 1 mm circle mean thickness (μ m) | 273.94 ± 25.27 | 400.20 ± 36.86 | 0.003 |
| Macular volume (mm3) | 8.25 ± 0.59 | 9.35 ± 0.37 | 0.493 |
| SRHRMT (μ m) | 36.26 ± 20.56 | 197.23 ± 22.69 | 0.001 |
| FT-SRHRMT (μ m) | 219.72 ± 123.83 | 175.44 ± 48.87 | 0.134 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download