Abstract
Purpose
Using a visual analogue scale, patients pain was compared according to injection site during intravitreal injection.
Methods
A prospective, clinical trial was conducted on 171 eyes of patients experiencing age-related macular degeneration, diabetic retinopathy, retinal vein occlusion, or central serous chorioretinopathy. After determining the anatomic quadrant of the injection site, patients were randomized to receive intravitreal bevacizumab, aflibercept, ranibizumab, or dexamethasone injection. Fifteen minutes after the injection, patients completed a survey about pain using a visual analogue scale from 0 (no pain) to 10 (unbearable pain).
Go to : 
References
1. Yau GL, Jackman CS, Hooper PL, Sheidow TG. Intravitreal abdominal anesthesia-comparison of different topical agents: a prospective randomized controlled trial. Am J Ophthalmol. 2011; 151:333–7.e2.
2. Gunther JB, Altaweel MM. Bevacizumab (Avastin) for the abdominal of ocular disease. Surv Ophthalmol. 2009; 54:372–400.
3. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–31.

4. Varma R, Bressler NM, Suñer I, et al. Improved vision-related function after ranibizumab for macular edema after retinal vein abdominal: results from the BRAVO and CRUISE trials. Ophthalmology. 2012; 119:2108–18.
5. Diabetic Retinopathy Clinical Research Network. Elman MJ, Aiello LP, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for abdominal macular edema. Ophthalmology. 2010; 117:1064–77.e35.
6. Peyman GA, Lad EM, Moshfeghi DM. Intravitreal injection of therapeutic agents. Retina. 2009; 29:875–912.

7. Bhavsar AR, Ip MS, Glassman AR. DRCRnet and the SCORE Study Groups. The risk of endophthalmitis following intravitreal triamcinolone injection in the DRCRnet and SCORE clinical trials. Am J Ophthalmol. 2007; 144:454–6.

8. Hoang QV, Mendonca LS, Della Torre KE, et al. Effect on abdominal pressure in patients receiving unilateral intravitreal antiabdominal endothelial growth factor injections. Ophthalmology. 2012; 119:321–6.
9. Sanabria MR, Montero JA, Losada MV, et al. Ocular pain after abdominal injection. Curr Eye Res. 2013; 38:278–82.
10. Eaton AM, Gordon GM, Wafapoor H, et al. Assessment of novel guarded needle to increase patient comfort and decrease injection time during intravitreal injection. Ophthalmic Surg Lasers Imaging Retina. 2013; 44:561–8.

11. Green-Simms AE, Ekdawi NS, Bakri SJ. Survey of intravitreal injection techniques among retinal specialists in the United States. Am J Ophthalmol. 2011; 151:329–32.

12. Güler M, Bilgin B, Çapkı n M, et al. Assessment of patient pain abdominal during intravitreal 27-gauge bevacizumab and 30-gauge ranibizumab injection. Korean J Ophthalmol. 2015; 29:190–4.
13. Rodrigues EB, Grumann A Jr, Penha FM, et al. Effect of needle type and injection technique on pain level and vitreal reflux in abdominal injection. J Ocul Pharmacol Ther. 2011; 27:197–203.
14. Knecht PB, Michels S, Sturm V, et al. Tunnelled versus straight abdominal injection: intraocular pressure changes, vitreous reflux, and patient discomfort. Retina. 2009; 29:1175–81.
15. Reed MD, Van Nostran W. Assessing pain intensity with the visual analog scale: a plea for uniformity. J Clin Pharmacol. 2014; 54:241–4.

16. Cousen P, Cackett P, Bennett H, et al. Tear production and corneal sensitivity in diabetes. J Diabetes Complications. 2007; 21:371–3.

17. Tavakoli M, Kallinikos PA, Efron N, et al. Corneal sensitivity is abdominal and relates to the severity of neuropathy in patients with diabetes. Diabetes Care. 2007; 30:1895–7.
Go to : 
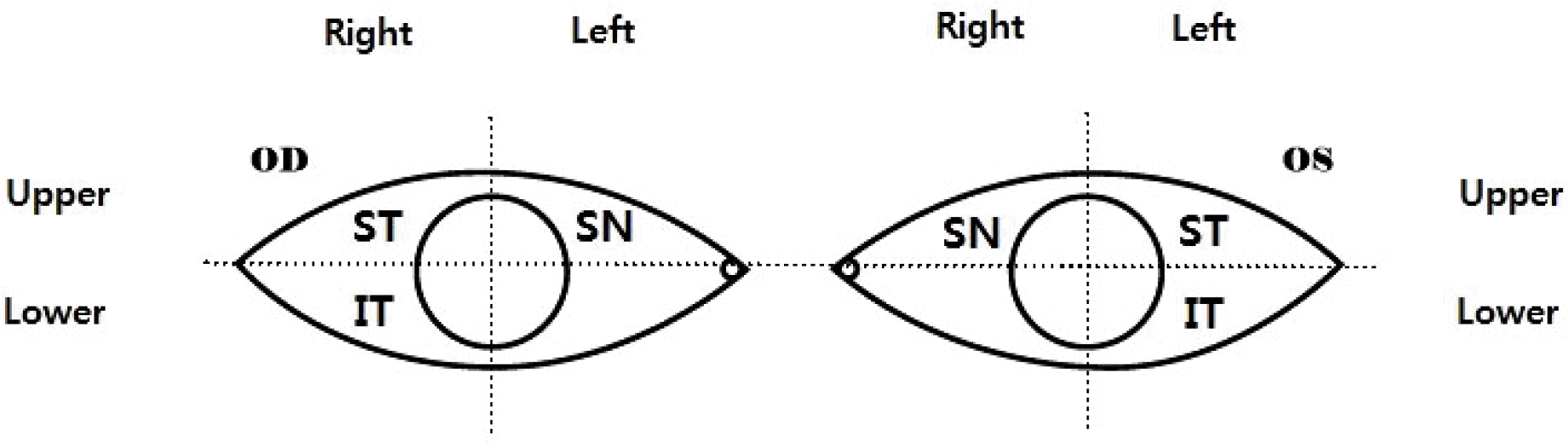 | Figure 1.Injection site description. ST = superotemporal; SN = superonasal; IT = inferotemporal. |
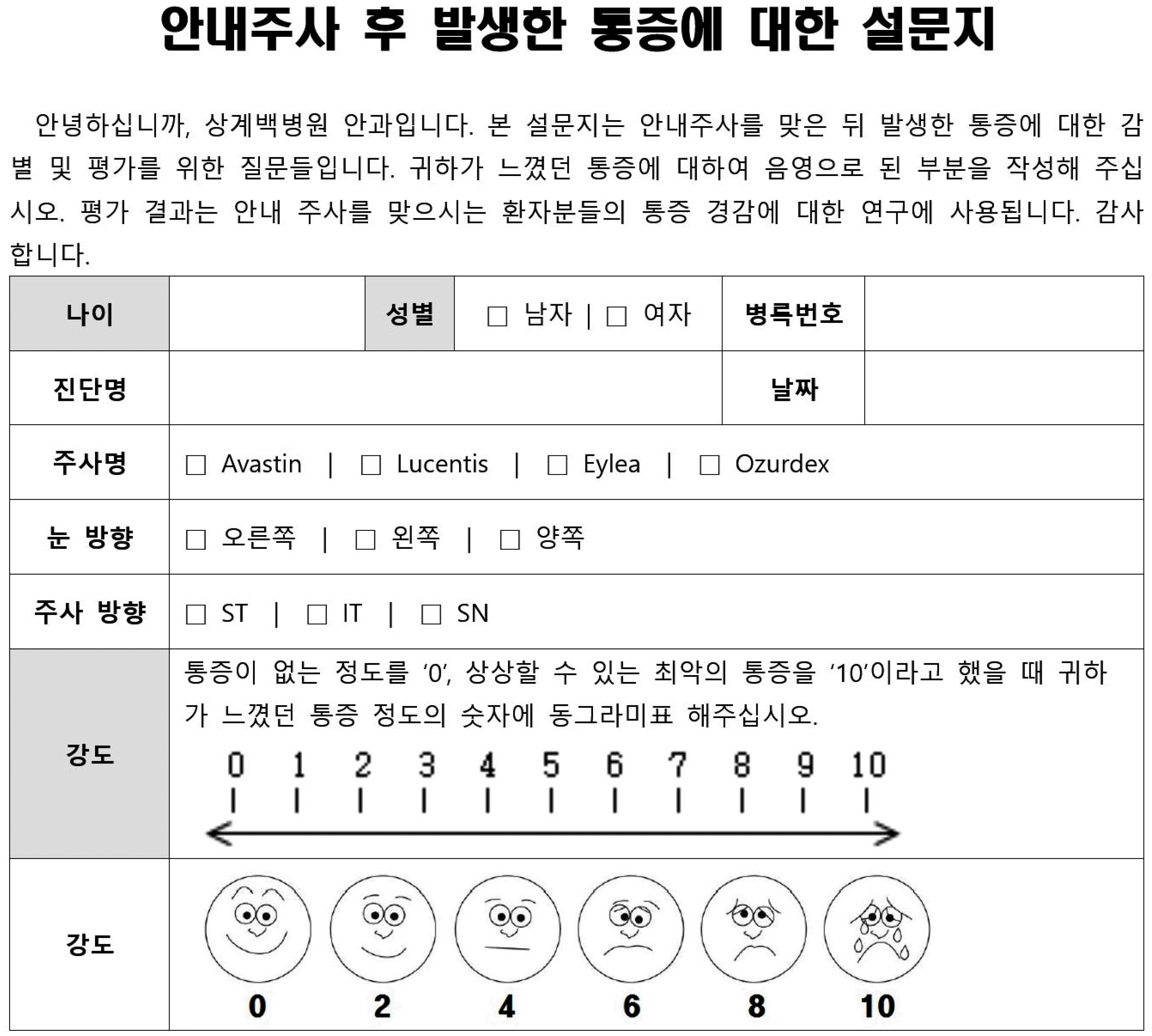 | Appendix 1.Questionnaire about pain occurred after intravitreal injection. ST = superotemporal; IT = inferotemporal; SN = superonasal. |
Table 1.
Comparison of pain after intravitreal injection
| Mean pain (SD) | p-value | |
|---|---|---|
| Age | ||
| ≥65 year | 3.13 (1.81) | 0.008* |
| <65 year | 2.35 (1.79) | |
| Sex | ||
| Male | 2.73 (1.85) | 0.272* |
| Female | 3.04 (1.82) | |
| Past history | ||
| Diabetes mellitus (+) | 2.17 (1.45) | 0.011* |
| Diabetes mellitus (−) | 3.05 (1.88) | |
| Drug | ||
| Bebacizumab | 2.66 (1.70) | 0.607† |
| Aflibercept | 2.95 (1.96) | |
| Ranibizumab | 3.09 (1.86) | |
| Dexamethasone | 3.60 (2.70) | |
| Injection site | ||
| Superotemporal | 3.20 (1.78) | 0.012† |
| Superonasal | 3.03 (1.79) | |
| Inferotemporal | 2.35 (1.86) | |
| Right | 2.80 (1.96) | 0.607* |
| Left | 2.94 (1.71) | |
| Upper | 3.16 (1.80) | 0.002* |
| Lower | 2.25 (1.77) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download