Abstract
Purpose
The aim of this study was to evaluate the clinical effect of 3% diquafosol in dry eye patients aged around 60 years.
Methods
In total, 68 patients with dry eye syndrome were divided by age into 2 groups, Group I (29 patients, 29 eyes) under the age of 60 years and Group II (39 patients, 39 eyes) over the age of 60 years. To evaluate the effectiveness of 3% diquafosol, we measured the tear film break-up time (tBUT), performed the Schirmer I test, and used the corneal staining score as an objective indicator and the ocular surface disease index (OSDI) score as a subjective indicator at initial visit, 1 month, 2 months, and 4 months.
Results
Significant improvements in the tear film break-up time, Schirmer I test, and OSDI were observed at 1,2, and 4 months after treatment with 3% diquafosol tetrasodium in both dry eye groups, but significant difference in the corenal staining score were not observed (p > 0.05). There were statistically significant improvement between the 2 age groups in the tBUT at 1 month (p = 0.012), 2 months (p = 0.005), and 4 months (p = 0.005), and improvements in the Schirmer I test between the 2 age groups at 1 month (p = 0.015), 2 months (p = 0.005), and 4 months (p = 0.005) were also observed. But, there was no significant difference in the corneal staining score and OSDI score between the 2 groups at 1,2, and 4 months (p > 0.05).
Go to : 
References
1. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007). Ocul Surf. 2007; 5:75–92.
2. Paulsen AJ. Cruickshanks KJ. Fischer ME, et al. Dry eye in the beaver dam offspring study: prevalence, risk factors and health-related quality of life. Am J Ophthalmol. 2014; 157:799–806.

3. Damasceno RW. Oksaki MH. Dantas PE. Belfort R Jr.Involutional entropion and ectropion of the lower eyelid: prevalence and associated risk factors in the elderly population. Ophthal Plast Reconstr Surg. 2011; 27:317–20.

4. Sullivan DA. Jensen RV. Suzuki T. Richards SM. Do sex steroids exert sex-specific and/or opposite effects on gene expression in lacrimal and meibomian glands? Mol Vis. 2009; 15:1553–72.
5. Tsubota K. Kawashima M. Inaba T, et al. The antiaging approach for the treatment of dry eye. Cornea. 2012; 31:1. S3–8.

6. Foulks GN. Pharmacological management of dry eye in the elderly patient. Drugs Aging. 2008; 25:105–18.

7. Fujihara T. Murakami T. Fujita H, et al. Improvement of corneal barrier function by the P2Y(2) agonist INS365 in a rat dry eye model. Invest Ophthalmol Vis Sci. 2001; 42:96–100.
8. Matsumoto Y. Ohashi Y. Watanabe H, et al. Efficacy and safety of diquafosol ophthalmic solution in patients with dry eye syndrome: a Japanese phase 2 clinical trial. Ophthalmology. 2012; 119:1954–60.

9. Kamiya K. Nakanishi M. Ishii R, et al. Clinical evalution of the additive effect of diquafosol tetrasodium on sodium hyaluronate monotherapy in patients with dry eye syndrome: a prospective, randomized, multicenter study. Eye (Lond). 2012; 26:1363–8.
10. Takamura E. Tsubota K. Watanabe H, et al. A randomised, double-masked comparison study of diquafosol versus sodium hyaluronate ophthalmic solutions in dry eye patients. Br J Ophthalmol. 2012; 96:1310–5.

11. Kaido M. Uchino M. Kojima T, et al. Effects of diquafosol tetrasodium administration on visual function in short break-up time dry eye. J Ocul Pharmacol Ther. 2013; 29:595–603.

12. Shimazaki-Den S. Iseda H. Dogru M, et al. Effects of diquafosol sodium eye drops on tear film stability in short BUT type of dry eye. Cornea. 2013; 32:1120–5.

13. Jung HH. Kang YS. Sung MS. Yoon KC. Clinical efficacy of topical 3% diquafosol tetrasodium in short tear film break-up time dry eye. J Korean Ophthalmol Soc. 2015; 56:339–44.

14. Lemp MA. Crews LA. Bron AJ, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012; 31:472–8.
15. Yoon KC. Im SK. Kim HG. You IC. Usefulness of double vital staining with 1% fluorescein and 1% lissamine green in patients with dry eye syndrome. Cornea. 2011; 30:972–6.

17. Kaido M. Goto E. Dogru M. Tsubota K. Punctal occlusion in the management of chronic Stevens-Johnson syndrome. Ophthalmology. 2004; 111:895–900.

18. Miller KL. Walt JG. Mink DR, et al. Minimal clinically important difference for the ocular surface disease index. Arch Ophthalmol. 2010; 128:94–101.

19. Yamaguchi M. Nishijima T. Shimazaki J, et al. Clinical usefulness of diquafosol for real-world dry eye patients: a prospective, open-label, non-interventional, observational study. Adv Ther. 2014; 31:1169–81.

20. Koh S. Ikeda C. Takai Y, et al. Long-term results of treatment with diquafosol ophthalmic solution for aqueous-deficient dry eye. Jpn J Ophthalmol. 2013; 57:440–6.

21. Roszkowska AM. Colosi P. Ferreri FM. Galasso S. Age-related modifications of corneal sensitivity. Ophthalmologica. 2004; 218:350–5.

23. Gong L. Sun X. Ma Z, et al. A randomised, parallel-group comparison study of diquafosol ophthalmic solution in patients with dry eye in China and Singapore. Br J Ophthalmol. 2015; 99:903–8.

24. Yamaguchi M. Nishijima T. Shimazaki J, et al. Real-world assessment of diquafosol in dry eye patients with risk factors such as contact lens, meibomian gland dysfunction, and conjunctivochalasis: subgroup analysis from a prospective observational study. Clin Ophthalmol. 2015; 9:2251–6.
25. Nichols KK. Mitchell GL. Zadnik K. The repeatability of clinical measurements of dry eye. Cornea. 2004; 23:272–85.

26. Tomlinson A. Khanal S. Ramaesh K, et al. Tear film osmolarity: determination of a referent for dry eye diagnosis. Invest Ophthalmol Vis Sci. 2006; 47:4309–15.

27. Zhang X. Chen W. De Paiva CS, et al. Interferon-y exacerbates dry eye-induced apoptosis in conjunctiva through dual apoptotic pathways. Invest Ophthalmol Vis Sci. 2011; 52:6279–85.
28. Gu Q. Dillon CF. Burt VL. Prescription drug use continues to increase: US. prescription drug data for 2007-2008. NCHS Data Brief. 2010; 42:1–8.

29. Somogyi A. Hewson D. Muirhead M. Bochner F. Amiloride disposition in geriatric patients: importance of renal function. Br J Clin Pharmacol. 1990; 29:1–8.

30. Zemba M. Papadatu CA. Enache VE. Sârbu LN. Ocular surface in glaucoma patients with topical treatment. Oftalmologia. 2011; 55:94–98.
31. Mangoni AA. Jackson SH. Age related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004; 57:6–14.
Go to : 
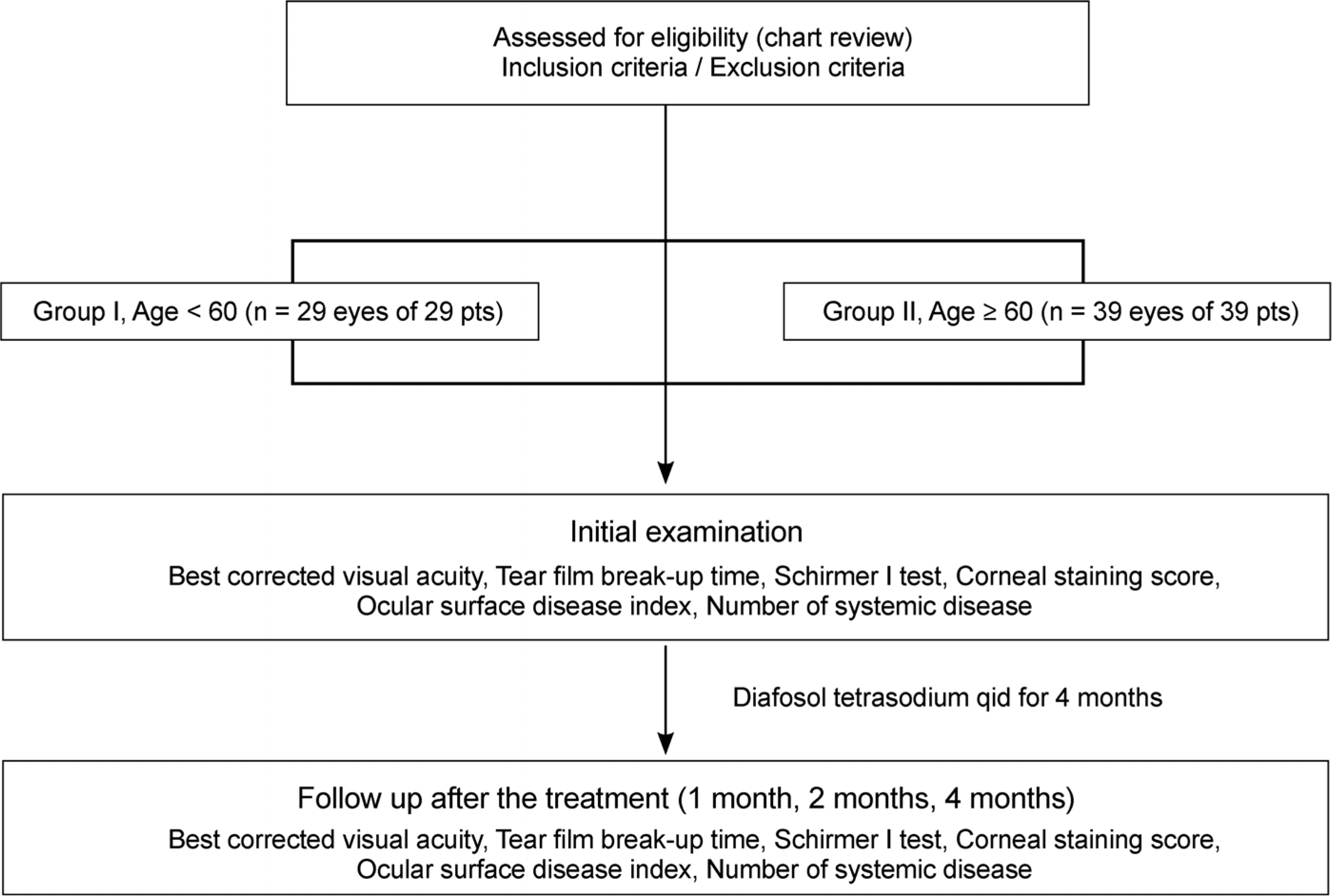 | Figure 1.Flow chart of the study for the therapeutic effects of topical 3% diquafosol tetrasodium. |
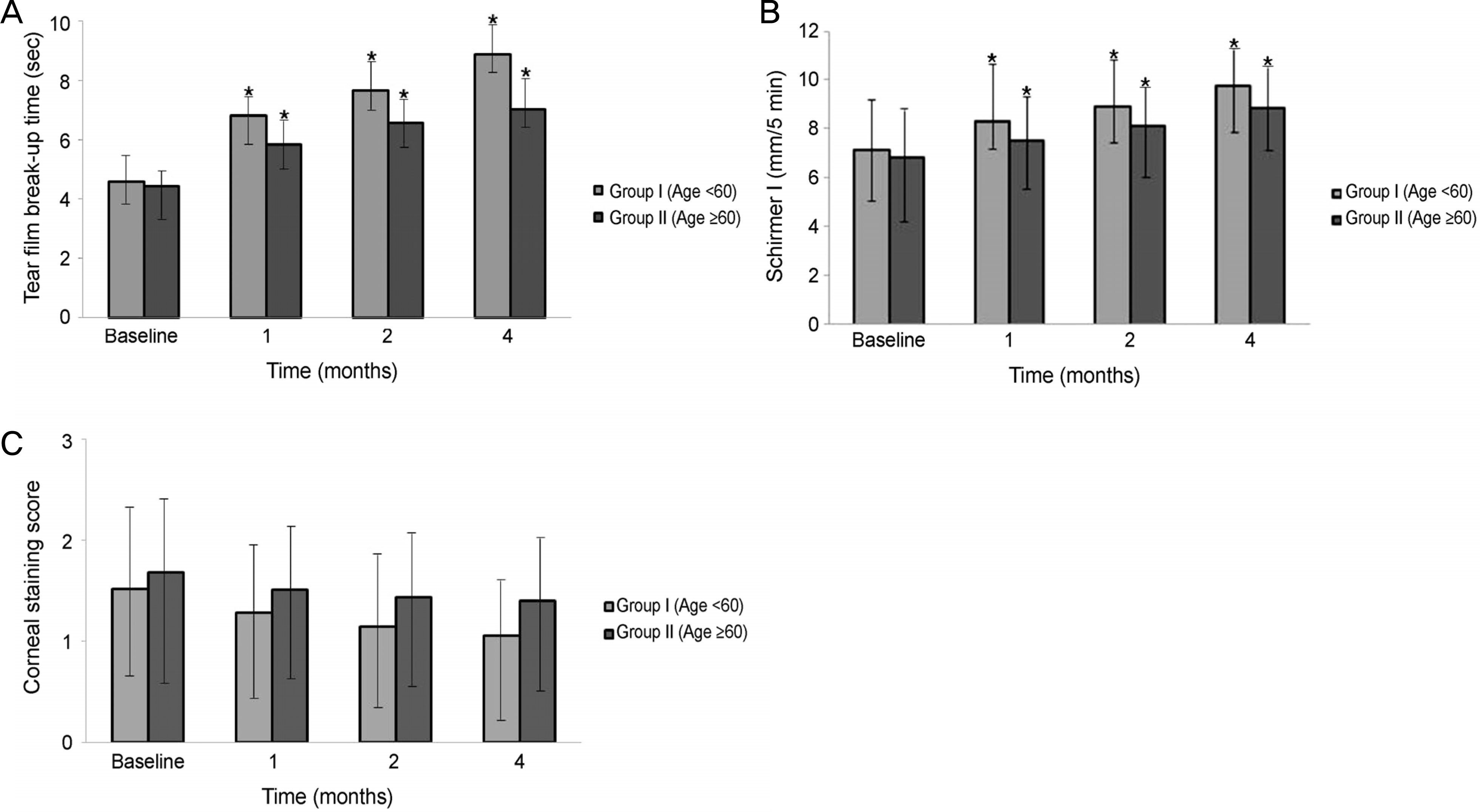 | Figure 2.Tear film and ocular surface parameters before and after treatment with topical 3% diquafosol tetrasodium. (A) Tear film break-up time, (B) Schirmer I score, (C) Corneal staining score. ∗p < 0.05 compared with baseline for each group. |
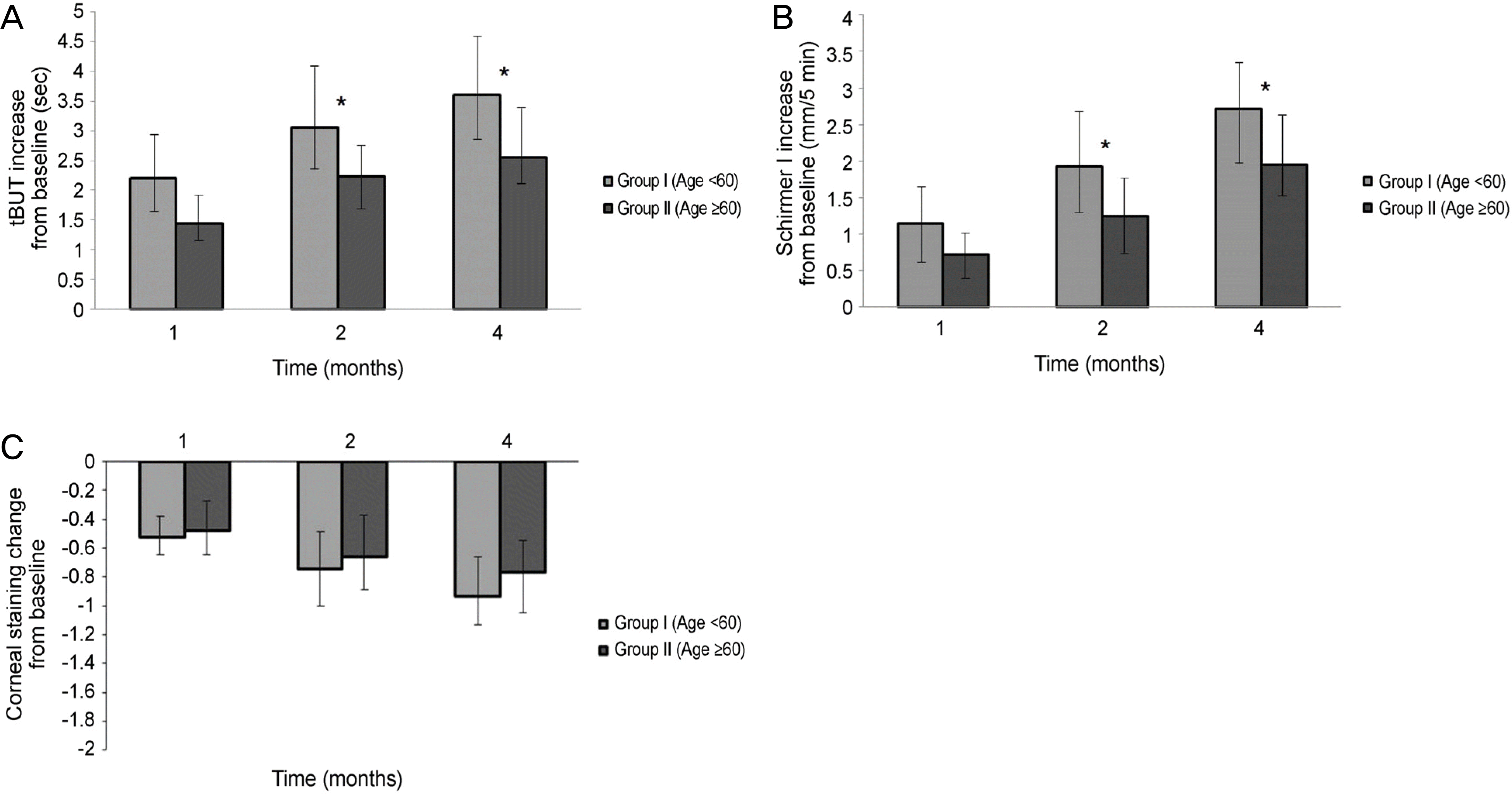 | Figure 3.Changes in tear film and ocular surface parameters after treatment with topical 3% diquafosol tetrasodium between two groups. (A) Tear film break-up time (tBUT), (B) Schirmer I score, (C) Corneal staining score. ∗p < 0.05 compared with group 1 and group 2. |
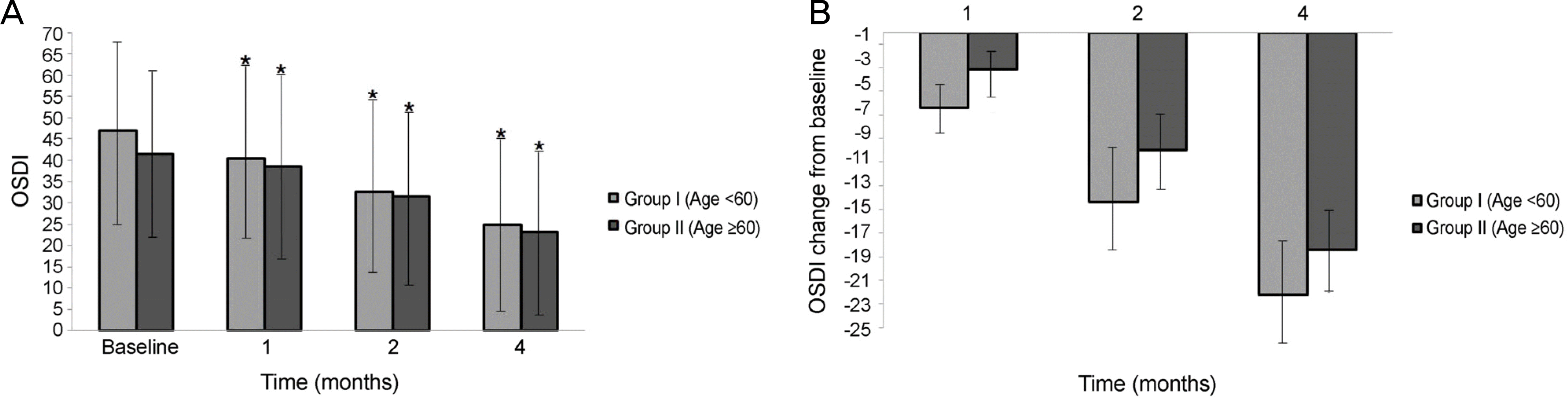 | Figure 4.Changes of ocular surface disease index (OSDI) score before and after treatment with topical 3% diquafosol tetrasodium. (A) Significant improvement in OSDI score was observed at 1, 2, and 4 months after treatment with 3% diquafosol tetrasodium for each group. (B) There was no significant difference in the OSDI score between two groups at 1, 2, and 4 months. ∗p < 0.05 compared with baseline for each group. |
Table 1.
Initial characteristics of each group with dry eye syndrome before treatment
| Group I (Age < 60) | Group II (Age ≥ 60) | p-value | |
|---|---|---|---|
| Total No. of patients (eyes) | 29 (29) | 39 (39) | |
| Sex (male/female) | 11/18 | 8/31 | |
| Mean age at diagnosis(years) | 51.93 ± 8.13 | 68.56 ± 5.11 | <0.001 |
| Mean BCVA at diagnosis (log MAR) | 0.09 ± 0.27 | 0.17 ± 0.25 | <0.001 |
| Corneal staining score | 1.52 ± 1.01 | 1.68 ± 1.04 | 0.260∗ |
| Tear film break-up time (sec) | 4.60 ± 0.65 | 4.43 ± 0.99 | 0.283∗ |
| Schirmer I test (mm) | 7.15 ± 2.43 | 6.82 ± 2.75 | 0.521∗ |
| Ocular surface disease index | 47.00 ± 23.59 | 41.49 ± 20.60 | 0.200∗ |
| Total No. of systemic disease | 0.83 ± 1.12 | 2.44 ± 1.56 | <0.001 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download