Abstract
Purpose
To evaluate the effect and prognostic factors of automated thermodynamic treatment (thermal compression therapy device [KCL 1100®]) for Meibomian gland dysfunction (MGD).
Methods
Patients (48 eyes of 24 subjects) with MGD were recruited for a prospective clinical trial. Patients received 15-minute treatments twice a day using the KCL 1100®. Severity of dry eye symptoms were evaluated using the Standard Patient Evaluation for Eye Dryness (SPEED) and Ocular Surface Disease Index (OSDI), and severity of Meibomian gland function was evaluated using the Meibomian gland expressibility (MGE), Meibomian gland secretion (MGS) score and lipid layer thickness measured by LipiView®. To evaluate ocular surface, we measured tear break-up time (BUT) and fluorescein corneal staining score (Oxford scale). Data were presented for baseline and at 2 weeks and 1 month post-treatment.
Results
Dry eye symptom (SPEED, OSDI), Meibomian gland function (MGE, MGS), and ocular surface index (BUT, Oxford scale) of patients were significantly improved from baseline to 2 weeks (p < 0.05) and 1 month post-treatment (p < 0.05). In addition, patients with more severe dry eye symptom and Meibomian gland index at baseline examination achieved improvement in mild to moderate MGD (p < 0.05). Improvement of Meibomian gland function (MGE) was associated with improvement of ocular surface index (BUT, Oxford scale) (p < 0.05), but not with improvement of dry eye symptom (SPEED, OSDI) (p > 0.05). There were no significant adverse events during the treatment.
Go to : 
References
1. Auw-Haedrich C. Reinhard T. Chronic blepharitis. Pathogenesis, clinical features, and therapy. Ophthalmologe. 2007; 104:817–26. quiz. 827–8.
2. Finis D. Schrader S. Geerling G. Meibomian gland dysfunction. Klin Monbl Augenheilkd. 2012; 229:506–13.
3. Geerling G. Tauber J. Baudouin C, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011; 52:2050–64.

4. Green-Church KB. Butovich I. Willcox M, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Invest Ophthalmol Vis Sci. 2011; 52:1979–93.

5. Knop E. Knop N. Meibomian glands: part IV. Functional interactions in the pathogenesis of meibomian gland dysfunction (MGD). Ophthalmologe. 2009; 106:980–7.
6. Knop E. Knop N. Millar T, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011; 52:1938–78.

7. McCulley JP. Shine WE. Meibomian secretions in chronic blepharitis. Adv Exp Med Biol. 1998; 438:319–26.

8. Nichols KK. Foulks GN. Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011; 52:1922–9.

9. Korb DR. Greiner JV. Increase in tear film lipid layer thickness following treatment of meibomian gland dysfunction. Adv Exp Med Biol. 1994; 350:293–8.

10. Key JE. A comparative study of eyelid cleaning regimens in chronic blepharitis. CLAO J. 1996; 22:209–12.
11. Smith RE. Flowers CW Jr.Chronic blepharitis: a review. CLAO J. 1995; 21:200–7.
13. Shine WE. McCulley JP. Meibomianitis: polar lipid abnormalities. Cornea. 2004; 23:781–3.
14. Goto E. Monden Y. Takano Y, et al. Treatment of non-inflamed obstructive meibomian gland dysfunction by an infrared warm compression device. Br J Ophthalmol. 2002; 86:1403–7.

15. Freedman HL. Preston KL. Heat retention in varieties of warm compresses: a comparison between warm soaks, hard-boiled eggs and the re-heater. Ophthalmic Surg. 1989; 20:846–8.

16. Blackie CA. Solomon JD. Greiner JV, et al. Inner eyelid surface temperature as a function of warm compress methodology. Optom Vis Sci. 2008; 85:675–83.

17. Kim DW. Kwon YA. Song SW. Clinical usefulness of a thermal-massaging system for treatment of dry eye with meibomian gland dysfunction. J Korean Ophthalmol Soc. 2013; 54:1321–6.

18. Finis D. Hayajneh J. König C, et al. Evaluation of an automated thermodynamic treatment (LipiFlow(R)) system for meibomian gland dysfunction: a prospective, randomized, observer-masked trial. Ocul Surf. 2014; 12:146–54.
19. Blackie CA. Solomon JD. Scaffidi RC, et al. The relationship between dry eye symptoms and lipid layer thickness. Cornea. 2009; 28:789–94.

20. Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5:108–52.
21. Bron AJ. Evans VE. Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003; 22:640–50.

22. Korb DR. Blackie CA. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea. 2008; 27:1142–7.

23. Benito A. Pérez GM. Mirabet S, et al. Objective optical assessment of tear-film quality dynamics in normal and mildly symptomatic dry eyes. J Cataract Refract Surg. 2011; 37:1481–7.

24. Matsumoto Y. Dogru M. Goto E, et al. Efficacy of a new warm moist air device on tear functions of patients with simple meibomian gland dysfunction. Cornea. 2006; 25:644–50.

25. Mori A. Shimazaki J. Shimmura S, et al. Disposable eyelid-warming device for the treatment of meibomian gland dysfunction. Jpn J Ophthalmol. 2003; 47:578–86.

26. Paranjpe DR. Foulks GN. Therapy for meibomian gland disease. Ophthalmol Clin North Am. 2003; 16:37–42.

27. Lane SS. DuBiner HB. Epstein RJ, et al. A new system, the Li pi Flow, for the treatment of meibomian gland dysfunction. Cornea. 2012; 31:396–404.
28. Blackie CA. Korb DR. Knop E, et al. Nonobvious obstructive meibomian gland dysfunction. Cornea. 2010; 29:1333–45.

29. Nelson JD. Shimazaki J. Benitez-del-Castillo JM, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011; 52:1930–7.

30. Arita R. Morishige N. Shirakawa R, et al. Effects of eyelid warming devices on tear film parameters in normal subjects and patients with meibomian gland dysfunction. Ocul Surf. 2015; 13:321–30.

31. Korb DR. Blackie CA. Case report: a successful LipiFlow treatment of a single case of meibomian gland dysfunction and dropout. Eye Contact Lens. 2013; 39:e1–3.
32. Finis D. König C. Hayajneh J, et al. Six-month effects of a thermodynamic treatment for MGD and implications of meibomian gland atrophy. Cornea. 2014; 33:1265–70.

33. Tesón M. Calonge M. Fernández I, et al. Characterization by Belmonte’s gas esthesiometer of mechanical, chemical, and thermal corneal sensitivity thresholds in a normal population. Invest Ophthalmol Vis Sci. 2012; 53:3154–60.

34. Hirata H. Meng ID. Cold-sensitive corneal afferents respond to a variety of ocular stimuli central to tear production: implications for dry eye disease. Invest Ophthalmol Vis Sci. 2010; 51:3969–76.

36. Su TY. Ho WT. Lu CY, et al. Correlations among ocular surface temperature difference value, the tear meniscus height, Schirmer’s test and fluorescein tear film break up time. Br J Ophthalmol. 2015; 99:482–7.

37. Hirata H. Rosenblatt MI. Hyperosmolar tears enhance cooling sensitivity of the corneal nerves in rats: possible neural basis for cold-induced dry eye pain. Invest Ophthalmol Vis Sci. 2014; 55:5821–33.

38. Vehof J. Kozareva D. Hysi PG, et al. Relationship between dry eye symptoms and pain sensitivity. JAMA Ophthalmol. 2013; 131:1304–8.

39. Belmonte C. Eye dryness sensations after refractive surgery: impaired tear secretion or “phantom” cornea? J Refract Surg. 2007; 23:598–602.

40. De Paiva CS. Pflugfelder SC. Corneal epitheliopathy of dry eye induces hyperesthesia to mechanical air jet stimulation. Am J Ophthalmol. 2004; 137:109–15.
Go to : 
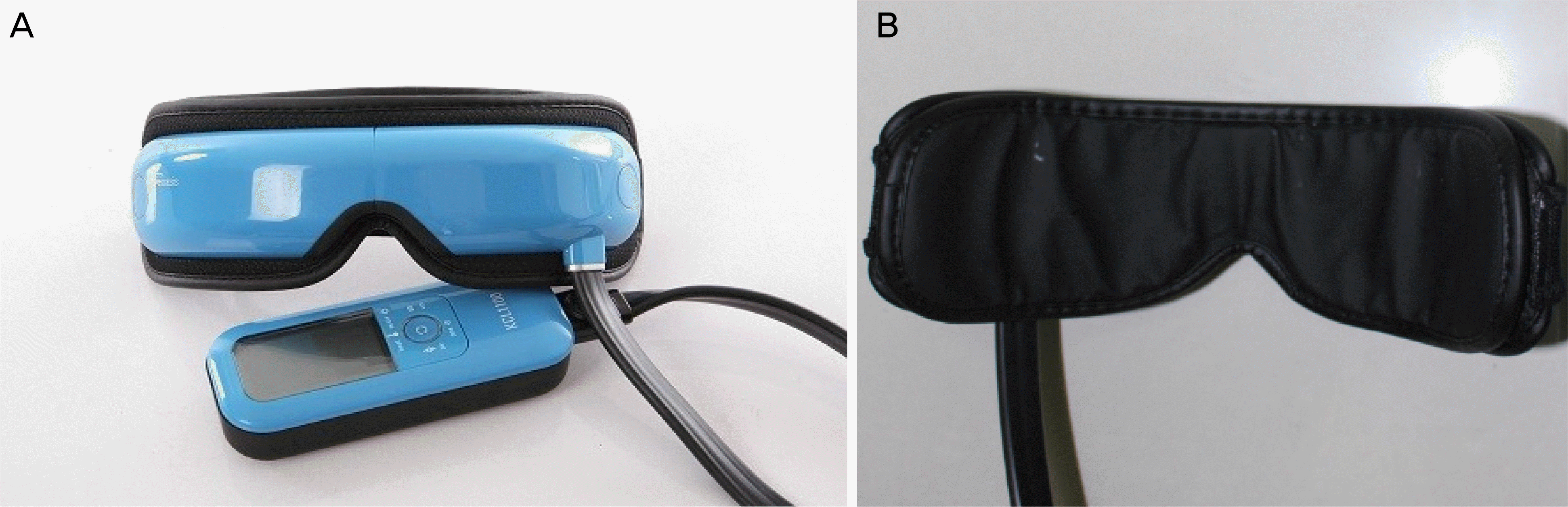 | Figure 1.Photographs of the thermal compression therapy device (KCL 1100®, Korea KCL, Bucheon, Korea). (A) The KCL 1100® is an automated thermodynamic treatment device system that generates heat and vibration, and massages the eyelid to treat Meibomian gland dysfunction (MGD). (B) Before the therapy, patients wore a moisturized cotton eye patch and then the device was used. |
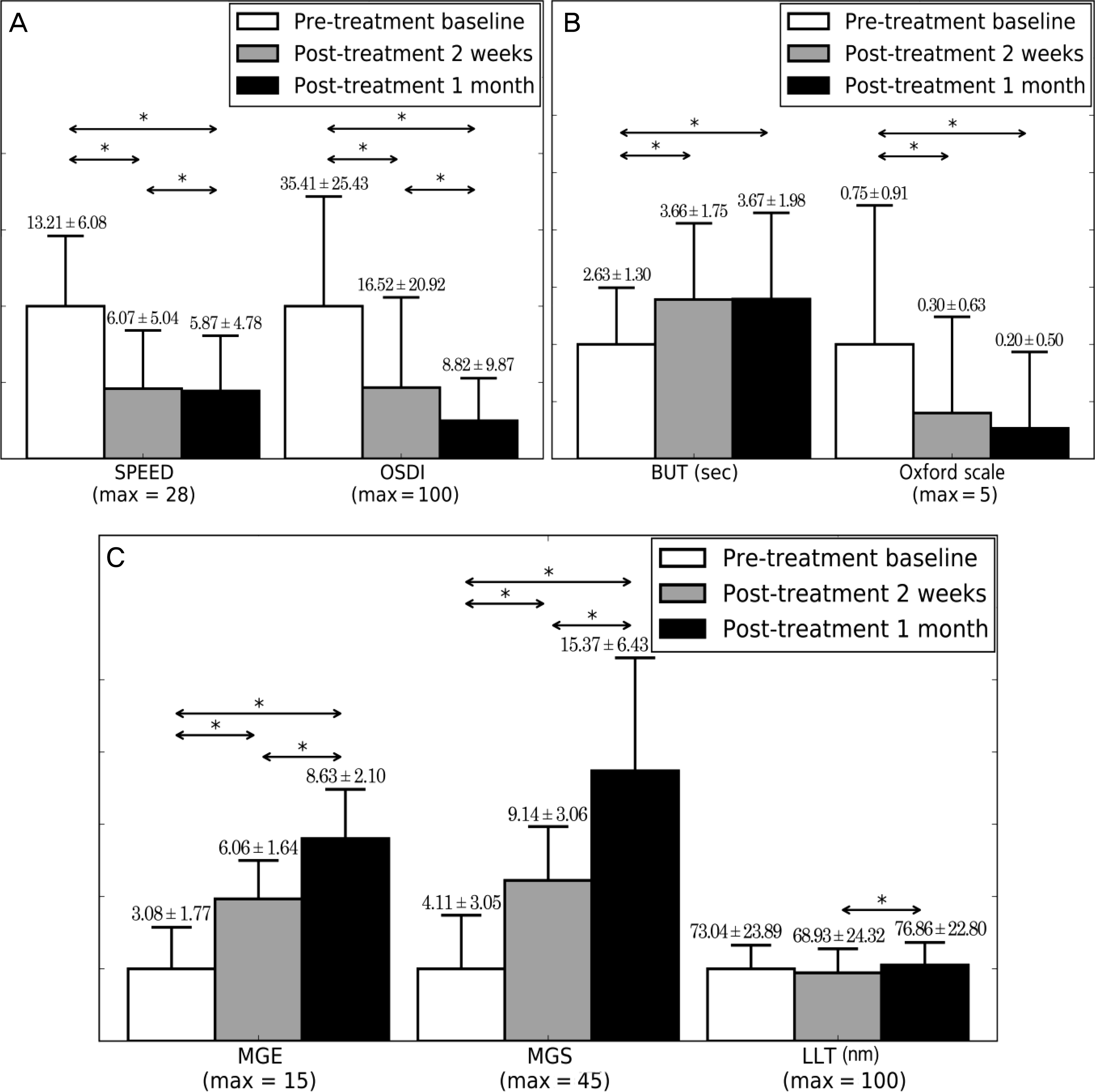 | Figure 2.Improvement of dry eye symptom, ocular surface and Meibomian gland index. (A) The mean Standard Patient Evaluation of Eye Dryness (SPEED) and Ocular Surface Disease Index (OSDI) at baseline, 2 weeks, and 1 month. (B) The mean tear break-up time (BUT) and fluorescein corneal stain score (Oxford scale) measured at baseline, 2 weeks and 1 month. (C) The mean Meibomian gland expressibility (MGE) and Meibomian gland secretion (MGS) score for the 15 Meibomian test glands of the lower eyelid and lipid layer thickness (LLT) using LipiView® (TearScience®, Morrisville, NC, USA) measured at baseline, 2 weeks and 1 month. Dry eye symptom (SPEED, OSDI), ocular surface index (BUT, Oxford scale), and Meibomian gland index (MGE, MGS) were significantly improved after 1 month of treatment with KCL 1100® (Korea KCL, Bucheon, Korea). ‘Significant (p < 0.05). |
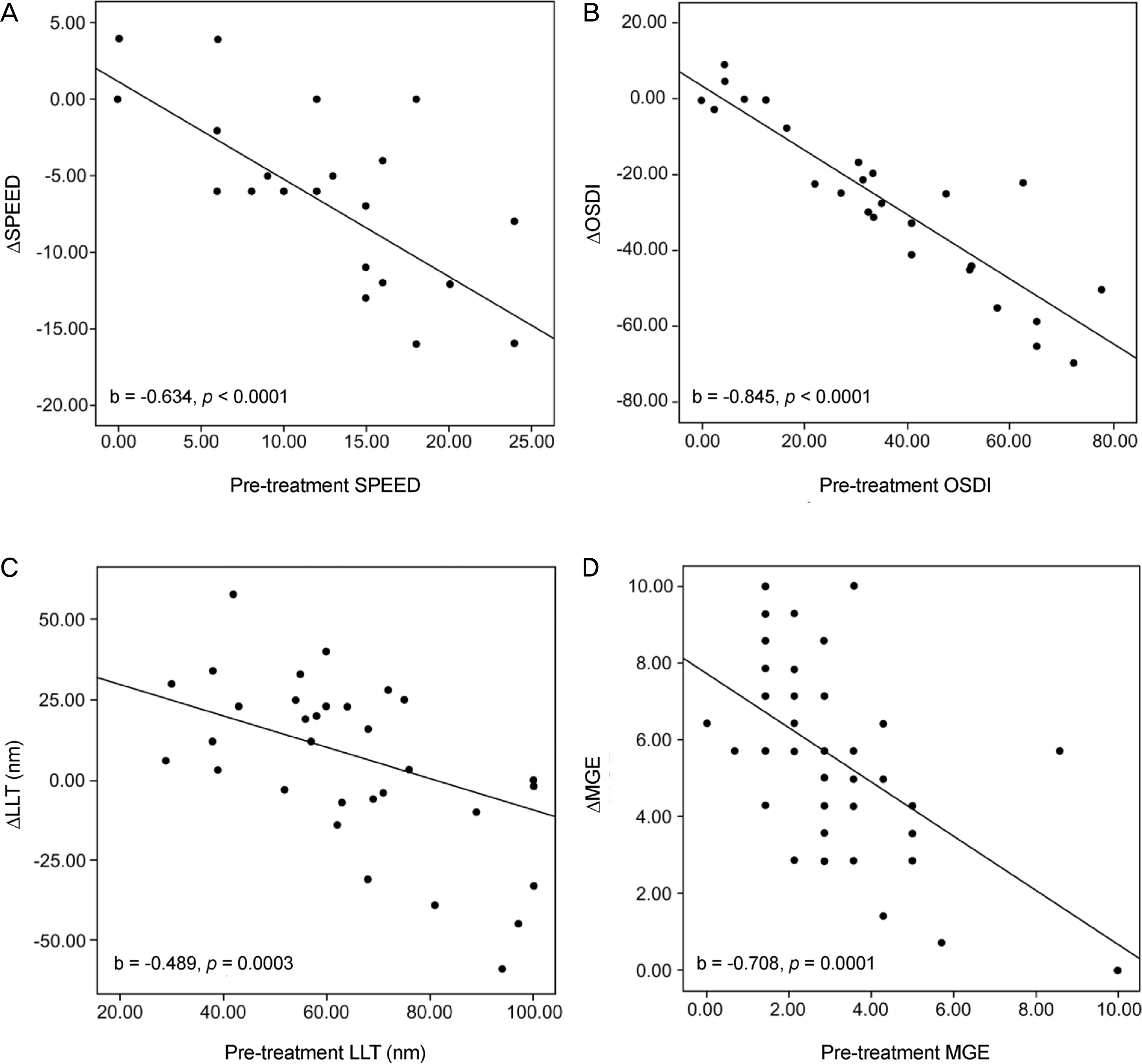 | Figure 3.Relationship between pre-treatment index and improvement during 1 month of thermodynamic treatment. (A, B) Scatter plots showed a negative correlation between initial value and change in dry eye symptom (Standard Patient Evaluation of Eye Dryness [SPEED], Ocular Surface Disease Index [OSDI]) and (C, D) Meibomian gland index (lipid layer thickness [LLT], Meibomian gland expressibility [MGE]). The patients with more severe dry eye symptom (SPEED, OSDI) and Meibomian gland index (LLT, MGE) at baseline examination achieved improvement with thermal compression therapy in mild to moderate Meibomian gland dysfunction (MGD). |
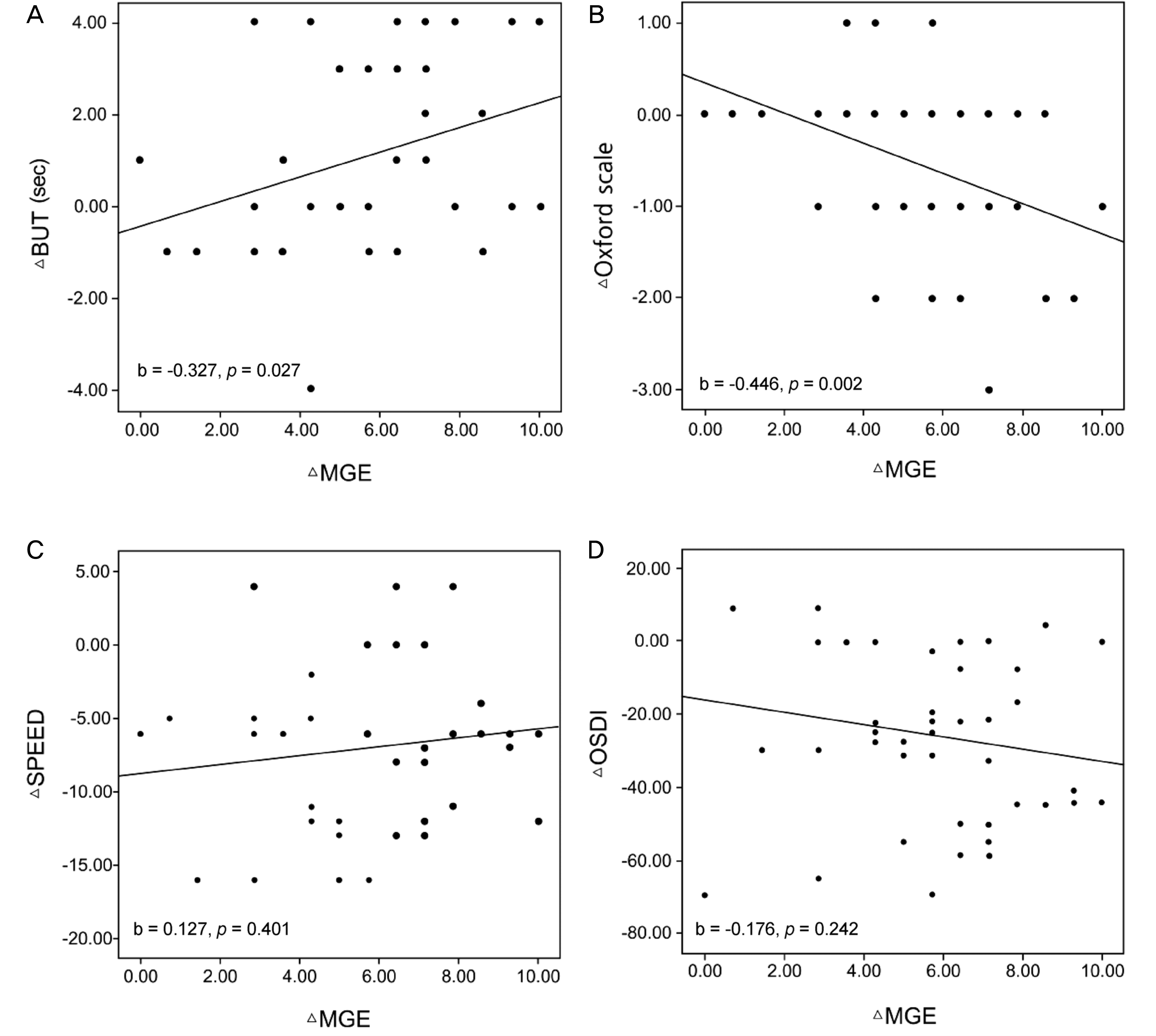 | Figure 4.Relationship between change of Meibomian gland opening and ocular surface index, and change in Meibomian gland opening and dry eye symptom. (A, B) Scatter plots showed a correlation between change in Meibomian gland opening (Meibomian gland expressibility [MGE]) and change in ocular surface index (tear breakup time (BUT), Oxford scale). (C, D) However, dry eye symptom (Standard Patient Evaluation of Eye Dryness [SPEED], Ocular Surface Disease Index [OSDI]) was not correlated with Meibomian gland opening (MGE). Meibomian gland opening (MGE) was associated with ocular surface index (BUT, Oxford scale), but not with dry eye symptom (SPEED, OSDI). |
Table 1.
Pretreatment demographics of patients who underwent automated thermodynamic therapy (48 eyes of 24 subjects)
| Characteristics | Value (range)∗ |
|---|---|
| Age | 61.9 ± 12.3 (22-79) |
| Sex (male:female) | 7:17 |
| SPEED | 13.2 ± 6.08 (0-24) |
| OSDI | 35.4 ± 25.4 (0.00-77.7) |
| MGE | 3.08 ± 1.77 (0-10) |
| MGS | 4.11 ± 3.05 (0-17) |
| LLT (nm) | 73.0 ± 23.9 (29.00-100.00) |
| BUT (sec) | 2.63 ± 1.30 (1.0-6.0) |
| Corneal staining score (Oxford scale) | 0.75 ± 0.91 (0-3) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download