Abstract
Purpose
To evaluate the effect of 0.05% cyclosporine A on the ocular surface after photorefractive keratectomy (PRK).
Methods
This retrospective study included 50 patients who underwent PRK. Patients were divided into two groups: 25 patients in group I were treated with topical 0.05% cyclosporine A with conventional medication, and 25 patients in group II were treated with conventional medication. Visual acuity (VA), tear break-up time (BUT), fluorescein staining score (F-stain), Schirmer I test, and ocular surface disease index (OSDI) were evaluated before surgery and 2 weeks, 1 month, 2 months, and 3 months after surgery.
References
1. Shin KH. Shyn KH. 2007 survey for KSCRS members-current trends in refractive surgery in Korea. J Korean Ophthalmol Soc. 2009; 50:1468–74.
2. Trattler WB. Barnes SD. Current trends in advanced surface ablation. Curr Opin Ophthalmol. 2008; 19:330–4.

3. Ghadhfan F. Al-Rajhi A. Wagoner MD. Laser in situ keratomileusis versus surface ablation: visual outcomes and complications. J Cataract Refract Surg. 2007; 33:2041–8.

4. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5:75–92.
5. Cho YK. Kim MS. Dry eye after cataract surgery and associated intraoperative risk factors. Korean J Ophthalmol. 2009; 23:65–73.

6. Aras C. Ozdamar A. Bahcecioglu H, et al. Decreased tear secretion after laser in situ keratomileusis for high myopia. J Refract Surg. 2000; 16:362–4.

7. Toda I. Asano-Kato N. Komai-Hori Y. Tsubota K. Dry eye after laser in situ keratomileusis. Am J Ophthalmol. 2001; 132:1–7.

8. Hong JW. Kim HM. The changes of tear break up time after myopic excimer laser photorefractive keratectomy. Korean J Ophthalmol. 1997; 11:89–93.

9. Lee JB. Ryu CH. Kim J, et al. Comparison of tear secretion and tear film instability after photorefractive keratectomy and laser in situ keratomileusis. J Cataract Refract Surg. 2000; 26:1326–31.

10. Lee BH. Kim EJ. Kim JH, et al. Changes in corneal sensation, tear film stability and ocular surface after advanced surface ablation. J Korean Ophthalmol Soc. 2013; 54:408–15.

11. Salib GM. McDonald MB. Smolek M. Safety and efficacy of cyclosporine 0.05% drops versus unpreserved artificial tears in dry-eye patients having laser in situ keratomileusis. J Cataract Refract Surg. 2006; 32:772–8.

12. Stern ME. Gao J. Siemasko KF, et al. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004; 78:409–16.

13. Horwath-Winter J. Vidic B. Schwantzer G. Schmut O. Early changes in corneal sensation, ocular surface integrity, and tear-film function after laser-assisted subepithelial keratectomy. J Cataract Refract Surg. 2004; 30:2316–21.

14. Nakamori K. Odawara M. Nakajima T, et al. Blinking is controlled primarily by ocular surface conditions. Am J Ophthalmol. 1997; 124:24–30.

15. Melki SA. Azar DT. LASIK complications: etiology, management, and prevention. Surv Ophthalmol. 2001; 46:95–116.
16. Ambrósio R Jr. Tervo T. Wilson SE. LASIK-associated dry eye and neurotrophic epitheliopathy: pathophysiology and strategies for prevention and treatment. J Refract Surg. 2008; 24:396–407.

17. Solomon A. Dursun D. Liu Z, et al. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001; 42:2283–92.
18. Wilson SE. Inflammation: a unifying theory for the origin of dry eye syndrome. Manag Care. 2003; 12:12. 14–9.
19. Luo L. Li DQ. Doshi A, et al. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004; 45:4293–301.

20. Kunert KS. Tisdale AS. Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002; 120:330–7.

21. Sall K. Stevenson OD. Mundorf TK. Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000; 107:631–9.
22. Brignole F. Pisella PJ. De Saint. Jean M, et al. Flow cytometric analysis of inflammatory markers in KCS: 6-month treatment with topical cyclosporin A. Invest Ophthalmol Vis Sci. 2001; 42:90–5.
23. Kunert KS. Tisdale AS. Stern ME, et al. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch Ophthalmol. 2000; 118:1489–96.
24. Peyman GA. Sanders DR. Batlle JF, et al. Cyclosporine 0.05% ophthalmic preparation to aid recovery from loss of corneal sensitivity after LASIK. J Refract Surg. 2008; 24:337–43.

25. Ursea R. Purcell TL. Tan BU, et al. The effect of cyclosporine A (Restasis) on recovery of visual acuity following LASIK. J Refract Surg. 2008; 24:473–6.

26. Lee HS. Jang JY. Lee SH, et al. Clinical effectiveness of topical cyclosporine a 0.05% after laser epithelial keratomileusis. Cornea. 2013; 32:e150–5.

27. Hessert D. Tanzer D. Brunstetter T, et al. Topical cyclosporine A for postoperative photorefractive keratectomy and laser in situ keratomileusis. J Cataract Refract Surg. 2013; 39:539–47.

28. Kang KW. Kim HK. Efficacy of topical cyclosporine in mild dry eye patients having refractive surgery. J Korean Ophthalmol Soc. 2014; 55:1752–7.

29. Tomás-Juan J. Murueta-Goyena Larrañaga A. Hanneken L. Corneal regeneration after photorefractive keratectomy: a review. J Optom. 2015; 8:149–69.

30. Donnenfeld ED. Solomon R. Roberts CW, et al. Cyclosporine 0.05% to improve visual outcomes after multifocal intraocular lens implantation. J Cataract Refract Surg. 2010; 36:1095–100.

31. Choi W. Yoon KC. Effect of 0.1% sodium hyaluronate and 0.05% cyclosporine on tear film parameters after cataract surgery. J Korean Ophthalmol Soc. 2011; 52:800–6.

32. Jeon JH. Kim HS. Jung JW, et al. Effect of cyclosporin A on tear film and corneal aberration after cataract surgery. J Korean Ophthalmol Soc. 2014; 55:978–83.

33. Pérez-Santonja JJ. Sakla HF. Cardona C, et al. Corneal sensitivity after photorefractive keratectomy and laser in situ keratomileusis for low myopia. Am J Ophthalmol. 1999; 127:497–504.

34. Murphy PJ. Corbett MC. O’Brart DP, et al. Loss and recovery of corneal sensitivity following photorefractive keratectomy for myopia. J Refract Surg. 1999; 15:38–45.
35. Herrmann WA. Shah CP. von Mohrenfels CW, et al. Tear film function and corneal sensation in the early postoperative period after LASEK for the correction of myopia. Graefes Arch Clin Exp Ophthalmol. 2005; 243:911–6.

36. Kim M. Chung SK. The changes of tear break-up time and schirmer’s test after photore fractive keratectomy. J Korean Ophthalmol Soc. 2001; 42:228–34.
Figure 1.
Comparison of uncorrected visual acuity between the groups over time. (A) Group I and group II show a significant difference compared to preoperative values during the follow-up period (compared with preoperative value by repeated measure analysis of variance [ANOVA]). (B) There was no statistically significant difference between the groups (compared by Mann-Whitney U-test). Group I = conventional treatment + 0.05% cyclosporine A; Group II = conventional treatment. ∗p < 0.05.
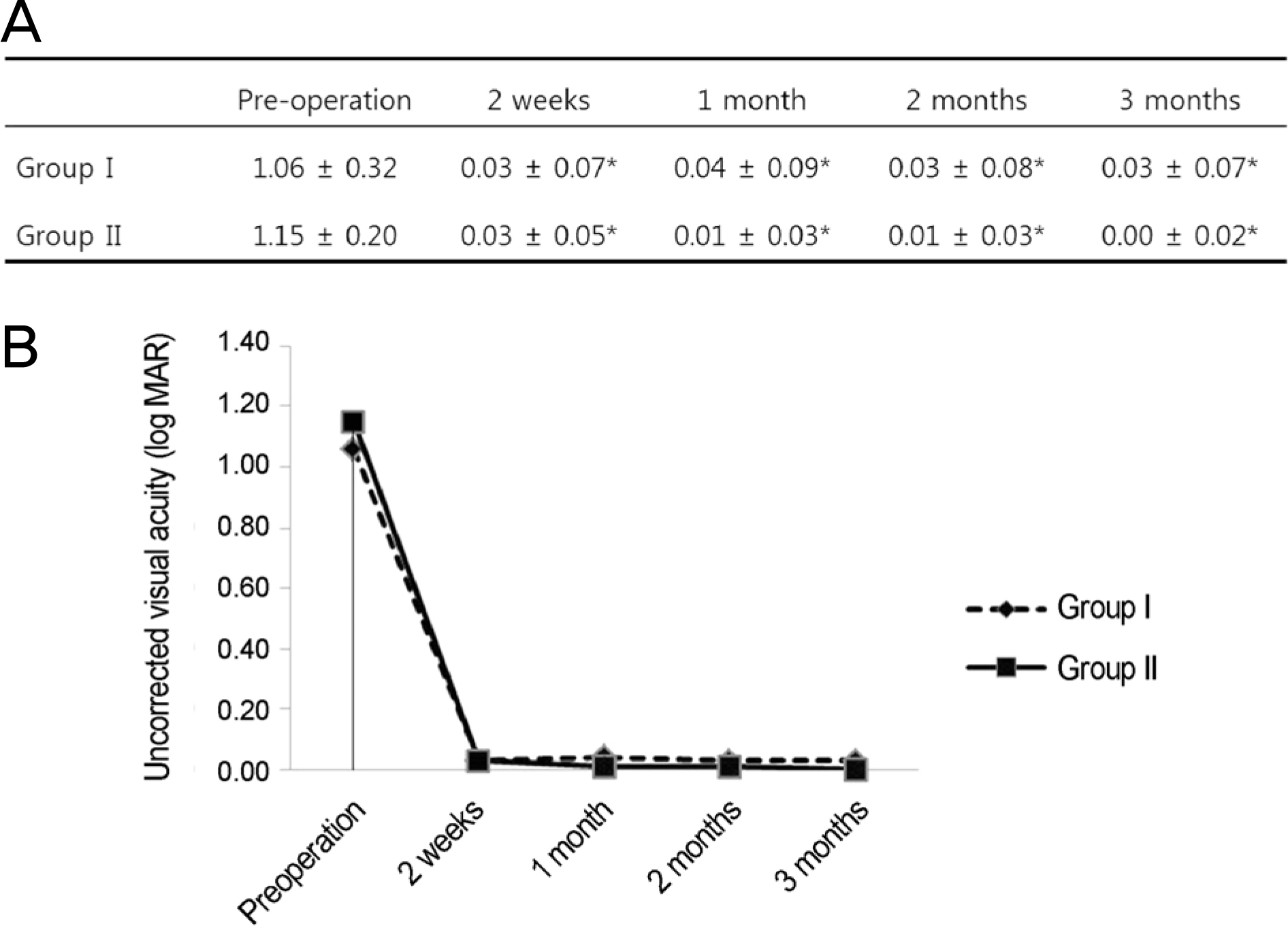
Figure 2.
Comparison of tear break-up time between the groups over time. (A) Group I shows a significant difference at 2 weeks compared with preoperative value, and group II at 2 weeks and 1 month (p < 0.05, compared with preoperative value by repeated measure analysis of variance [ANOVA]). (B) There is no statistically significant difference between the groups during the follow-up period (compared by Student’s t-test). Group I = conventional treatment + 0.05% cyclosporine A; Group II = conventional treatment. ∗p < 0.05.
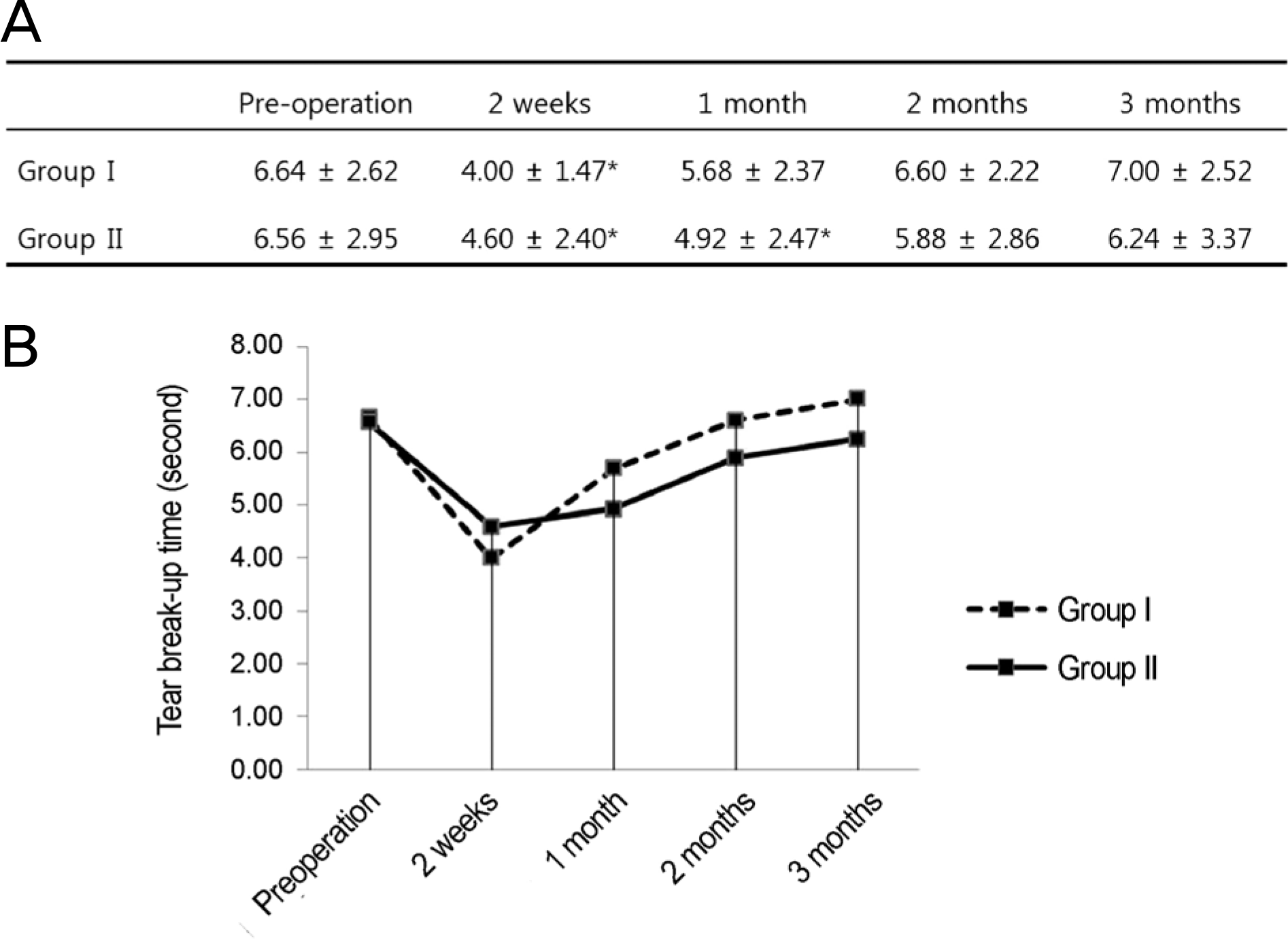
Figure 3.
Comparison of fluorescein staining score between the groups over time. (A) Group I shows a significant difference at 2 weeks compared with preoperative value, and group II at 2 weeks and 3 months (p < 0.05, compared with preoperative value by repeated measure analysis of variance [ANOVA]). (B) There is a statistically significant difference between the groups at 2 weeks and 1 month (p < 0.05, compared by Student’s t-test). Group I = conventional treatment + 0.05% cyclosporine A; Group II = conventional treatment. ∗p < 0.05.
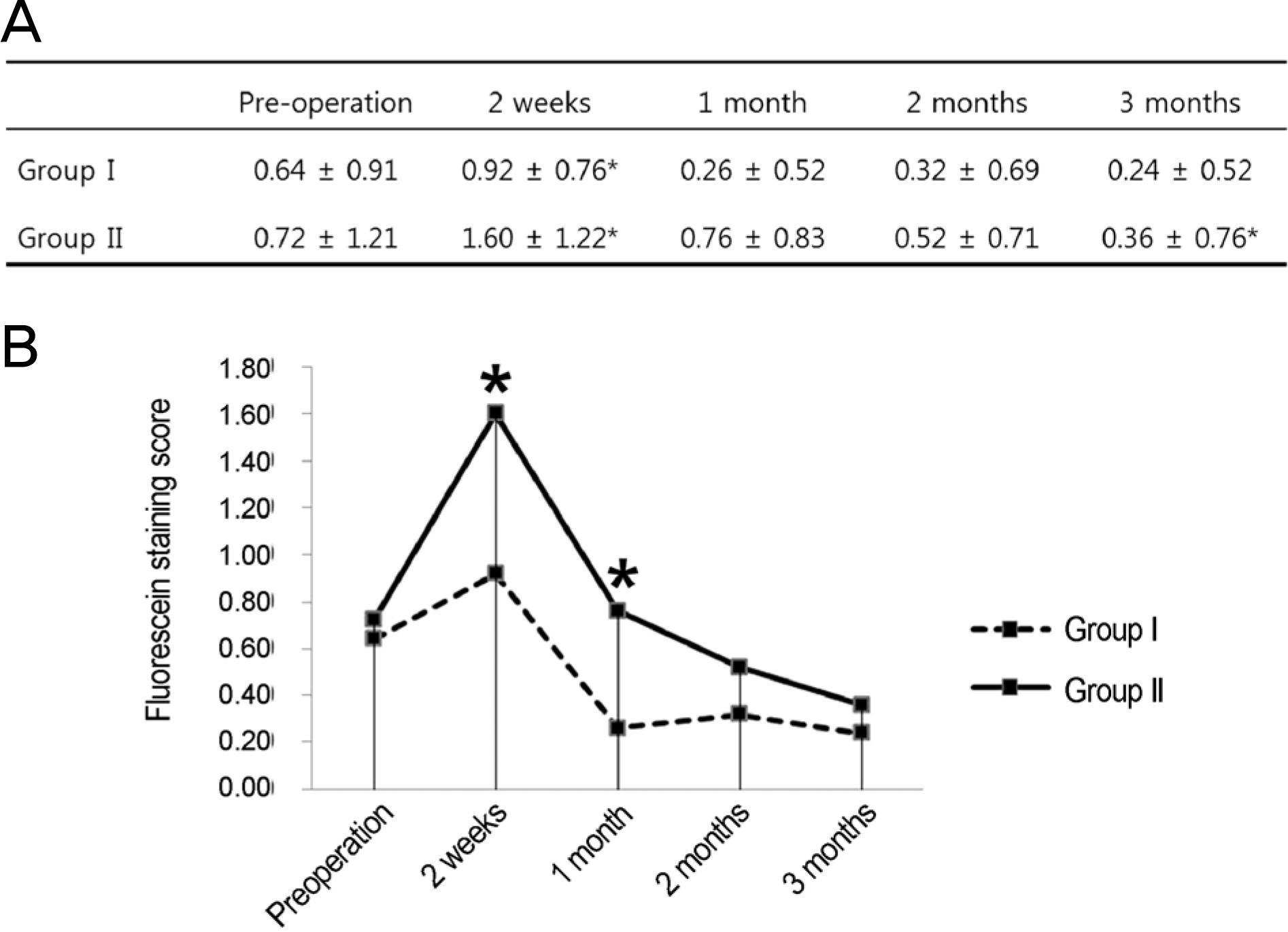
Figure 4.
Comparison of Schirmer I test between the groups over time. (A) Group I and group II shows no significant difference during the follow-up period compared with preoperative values (compared with preoperative value by repeated measure analysis of variance [ANOVA]). (B) There is no statistically significant difference between the groups during the follow-up period (compared by Student’s t-test). Group I = conventional treatment + 0.05% cyclosporine A; Group II = conventional treatment.
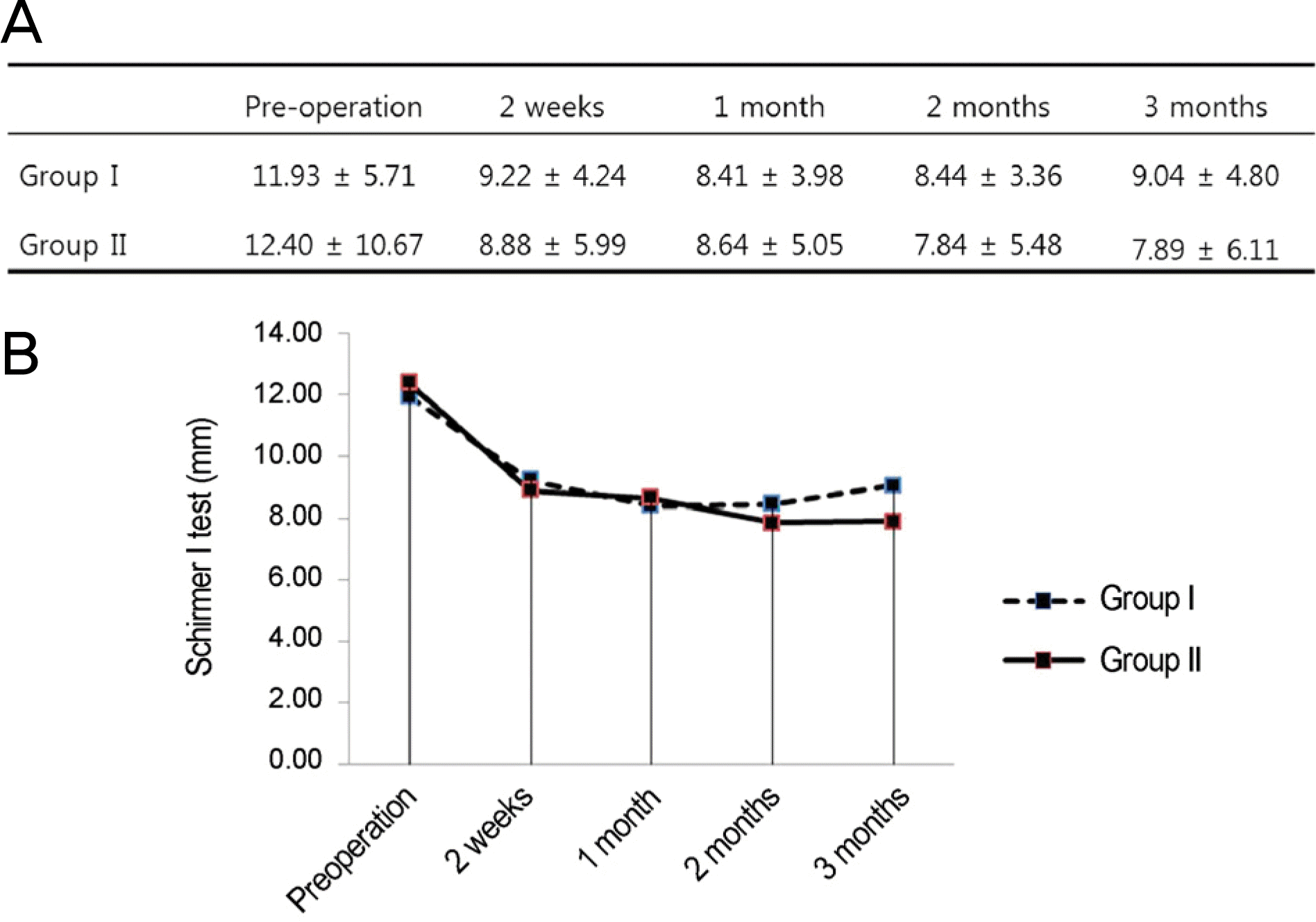
Figure 5.
Comparison of ocular surface disease index between the groups over time. (A) Group I and group II shows no significant difference during the follow-up period compared with preoperative value (compared with preoperative value by repeated measure analysis of variance [ANOVA]). (B) There is no statistically significant difference between the groups during the follow-up period (compared by Student’s t-test). Group = conventional treatment + 0.05% cyclosporine A; Group I = conventional treatment + 0.05% cyclosporine A; Group II = conventional treatment.
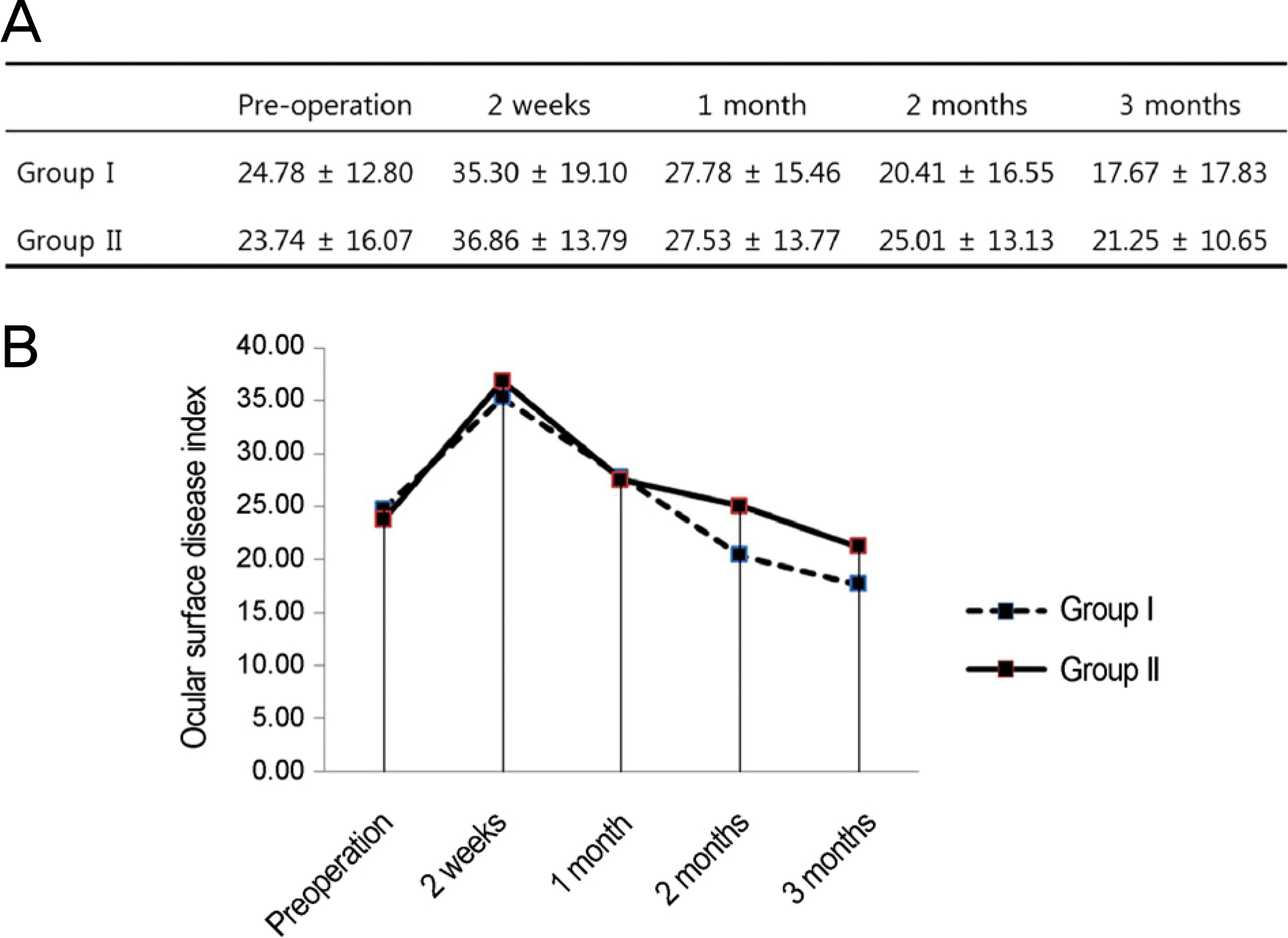
Table 1.
Demographics of the study groups
| Group I | Group II | p-value | |
|---|---|---|---|
| Number of eyes | 25 | 25 | |
| Patients (male:female) | 10:15 | 8:17 | 0.556∗ |
| Mean age (years) | 25.4 ± 6.6 | 25.8 ± 5.3 | 0.523† |
| Uncorrected visual acuity (log MAR) | 1.06 ± 0.32 | 1.15 ± 0.20 | 0.693† |
| Best corrected visual acuity (log MAR) | 0 | 0 | 1.000† |
| Spherical equivalent (diopter) | -4.86 ± 2.27 | -5.17 ± 2.45 | 0.352‡ |
| Ablation depth (μm) | 83.28 ± 30.70 | 87.33 ± 26.67 | 0.245‡ |
| Ablation diameter (mm) | 6.54 ± 0.30 | 6.45 ± 0.31 | 0.589‡ |
| Tear BUT (second) | 6.64 ± 2.62 | 6.56 ± 2.95 | 0.920‡ |
| F-stain | 0.64 ± 0.91 | 0.72 ± 1.21 | 0.792‡ |
| Schirmer I test (mm) | 11.93 ± 5.71 | 12.40 ± 10.67 | 0.832‡ |
| OSDI | 24.78 ± 12.80 | 23.74 ± 16.07 | 0.696‡ |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download