Abstract
Purpose
The aim of this study is to evaluate the effects and complications of mixed injections of botulinum neurotoxin A (BoNT-A), triamcinolone acetonide, 5-fluorouracil (5-FU) in patients with Graves upper eyelid retraction.
Methods
Twenty-four eyes of 17 patients with a mean age of 43.9 years showed symptoms of Grave’s upper eyelid retraction (GUER). They received mixed injections of BoNT-A 4 IU/0.1 mL, triamcinolone acetonide 4 mg/0.1 mL and 5-FU 5 mg/0.1 mL via subconjunctival injection. The response to treatment and the presence of adverse effects were followed up for 9.0 ± 6.0 months and evaluated retrospectively.
Results
Margin reflex distance 1 decreased significantly from 5.6 ± 1.2 mm to 4.7 ± 1.1 mm at 1 month after injection. Tarsal platform show increased significantly from 1.4 ± 1.3 mm to 1.8 ± 1.3 mm, and tear break up time increased significantly from 5.2 ± 3.1 seconds to 10.3 ± 7.8 seconds. When success was defined as the correction amount of GUER being larger than 1 mm, the success rate was 66.7%. Kaplan-Meier survival analysis showed that GUER correction effects last longer in patients with a duration of disease longer than 6 months. There were no severe adverse effects such as diplopia, blepharoptosis and intraocular pressure elevation.
Conclusions
Mixed injections of BoNT-A, triamcinolone acetonide and 5-FU, which compensate the side effects of solitary injection and enhances the anti-fibrotic effect, improves the eyelid position and tear film stability in the patients with GUER. It is an effective and safe method for treating GUER with long maintenance with less adverse effects.
Go to : 
References
1. Bartley GB. Fatourechi V. Kadrmas EF, et al. Clinical features of Graves’ ophthalmopathy in an incidence cohort. Am J Ophthalmol. 1996; 121:284–90.

2. Costa PG. Saraiva FP. Pereira IC, et al. Comparative study of Botox injection treatment for upper eyelid retraction with 6-month follow-up in patients with thyroid eye disease in the congestive or fibrotic stage. Eye (Lond). 2009; 23:767–73.

3. Garrity JA. Bahn RS. Pathogenesis of graves ophthalmopathy: implications for prediction, prevention, and treatment. Am J Ophthalmol. 2006; 142:147–53.

4. Bahn RS. Gorman CA. Choice of therapy and criteria for assessing treatment outcome in thyroid-associated ophthalmopathy. Endocrinol Metab Clin North Am. 1987; 16:391–407.

5. Cockerham KP. Hidayat AA. Brown HG, et al. Clinicopathologic evaluation of the Mueller muscle in thyroid-associated orbitopathy. Ophthal Plast Reconstr Surg. 2002; 18:11–7.

6. Morgenstern KE. Evanchan J. Foster JA, et al. Botulinum toxin type a for dysthyroid upper eyelid retraction. Ophthal Plast Reconstr Surg. 2004; 20:181–5.

7. Xu D. Liu Y. Xu H. Li H. Repeated triamcinolone acetonide injection in the treatment of upper-lid retraction in patients with thyroid-associated ophthalmopathy. Can J Ophthalmol. 2012; 47:34–41.

8. Uddin JM. Davies PD. Treatment of upper eyelid retraction associated with thyroid eye disease with subconjunctival botulinum toxin injection. Ophthalmology. 2002; 109:1183–7.
9. Jung BY. Kim YD. The results of periocular injections of triamcinolone for thyroid orbitopathy. J Korean Ophthalmol Soc. 2007; 48:1163–9.

10. Kim JE. Park JW. Cho JK. Yoon KC. Therapeutic effects of periocular injection of triamcinolon acetonide in patients with thyroid-associated ophthalmopathy. J Korean Ophthalmol Soc. 2011; 52:788–93.

11. Shih MJ. Liao SL. Lu HY. A single transcutaneous injection with Botox for dysthyroid lid retraction. Eye (Lond). 2004; 18:466–9.

12. Lee SJ. Rim TH. Jang SY, et al. Treatment of upper eyelid retraction related to thyroid-associated ophthalmopathy using subconjunctival triamcinolone injections. Graefes Arch Clin Exp Ophthalmol. 2013; 251:261–70.

13. Gebertt S. Depot-methylprednisolone for subconjunctival and retrobulbar injections. Lancet. 1961; 2:344–5.

14. Abraham LM. Selva D. Casson R. Leibovitch I. The clinical applications of fluorouracil in ophthalmic practice. Drugs. 2007; 67:237–55.

15. Gupta S. Kalra A. Efficacy and safety of intralesional 5-fluorouracil in the treatment of keloids. Dermatology. 2002; 204:130–2.

16. Yoo DB. Azizzadeh B. Massry GG. Injectable 5-FU with or without added steroid in periorbital skin grafting: initial observations. Ophthal Plast Reconstr Surg. 2015; 31:122–6.
17. Fitzpatrick RE. Treatment of inflamed hypertrophic scars using intralesional 5-FU. Dermatologic Surg. 1999; 25:224–32.

18. Terwee CB. Gerding MN. Dekker FW, et al. Development of a disease specific quality of life questionnaire for patients with Graves’ ophthalmopathy: the GO-QOL. Br J Ophthalmol. 1998; 82:773–9.

20. Burns CL. Gammon JA. Gemmill MC. Ptosis associated with botulinum toxin treatment of strabismus and blepharospasm. Ophthalmology. 1986; 93:1621–7.

21. Holds JB. Alderson K. Fogg SG. Anderson RL. Motor nerve sprouting in human orbicularis muscle after botulinum A injection. Invest Ophthalmol Vis Sci. 1990; 31:964–7.
22. Chee E. Chee SP. Subconjunctival injection of triamcinolone in the treatment of lid retraction of patients with thyroid eye disease: a case series. Eye (Lond). 2008; 22:311–5.

23. Renfro L. Snow JS. Ocular effects of topical and systemic steroids. Dermatol Clin. 1992; 10:505–12.

Go to : 
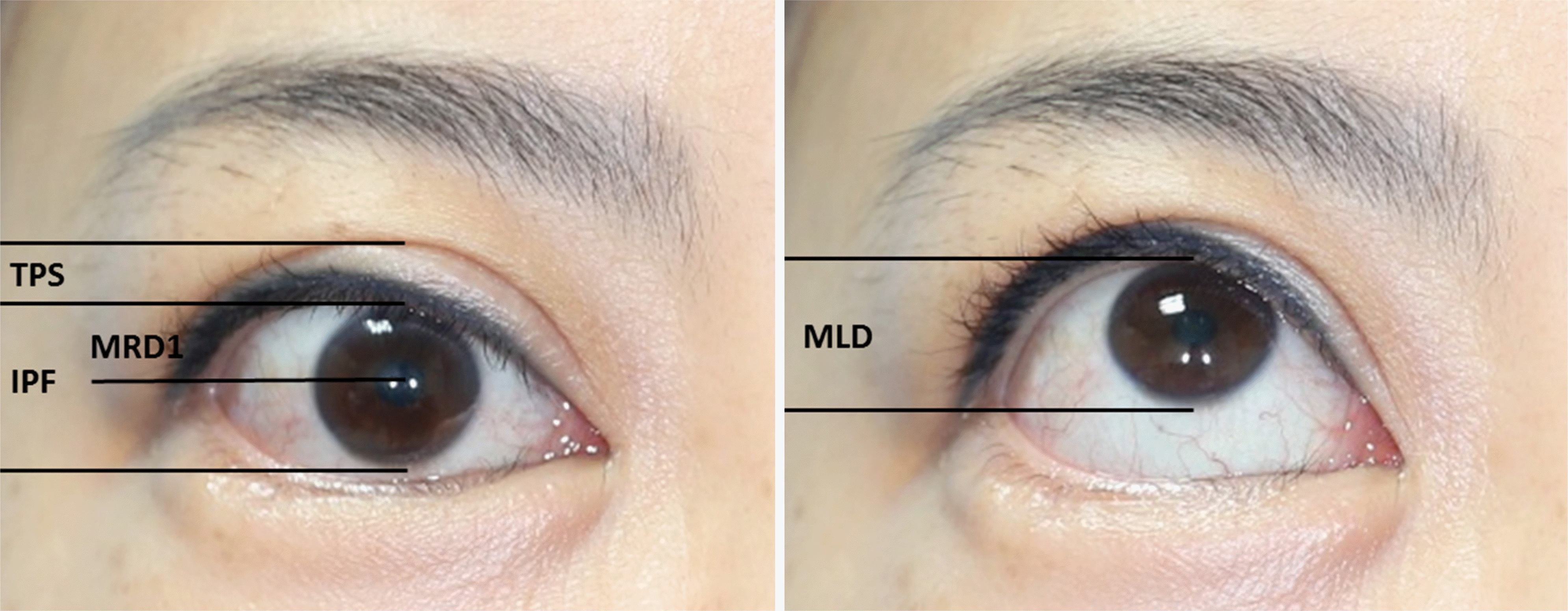 | Figure 1.Morphologic outcome measurement. MRD1 = margin reflex distance; IPF = interpalpebral fissure; TPS = tarsal platform show; MLD = margin limbal distance. |
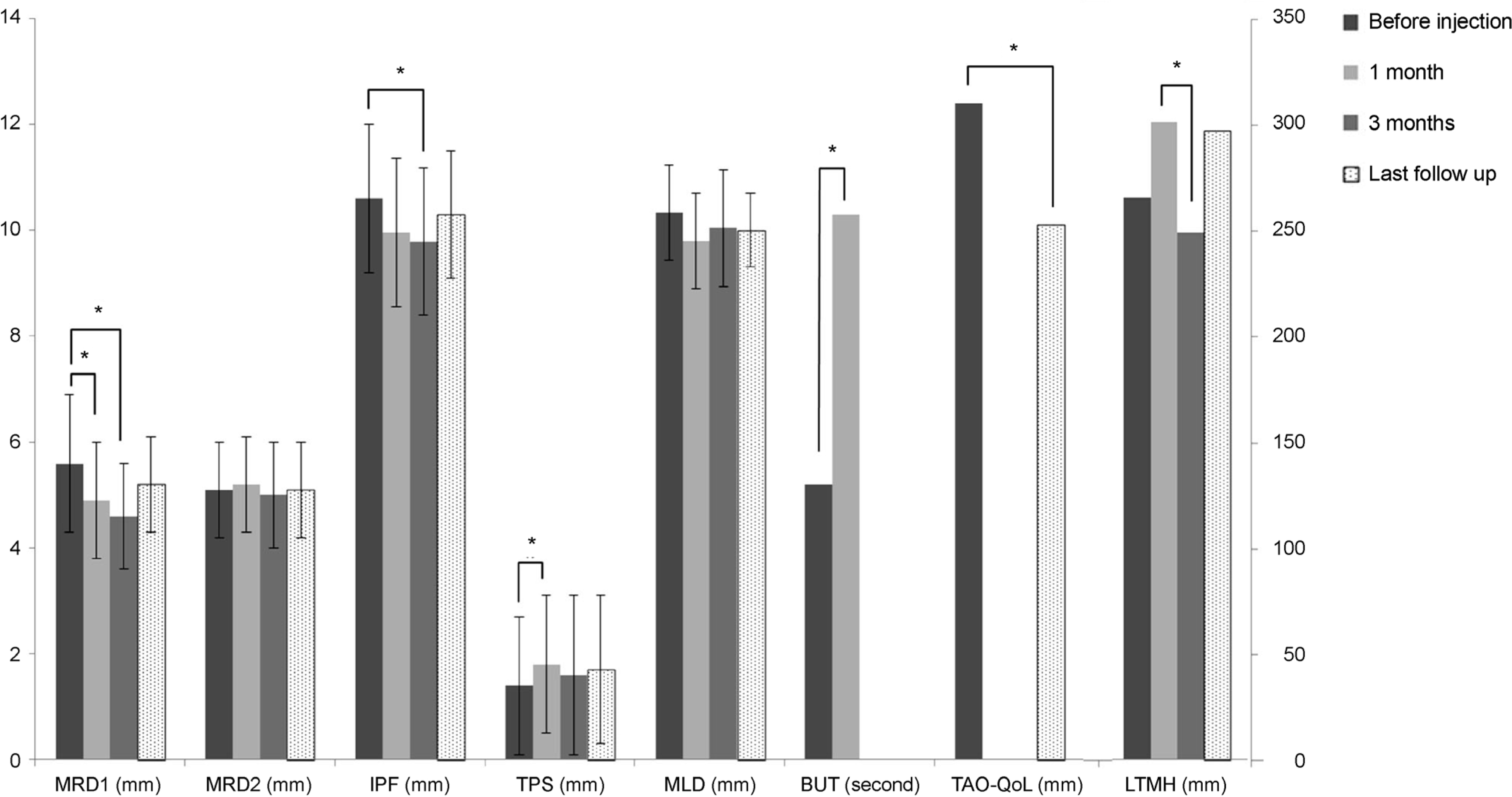 | Figure 2.Clinical outcomes after mixed injection of botulinum neurotoxin A (BoNT-A), triamcinolone and 5-fluorouracil (5-FU) in Graves’ upper eyelid retraction. MRD = margin reflex distance; IPF = interpalpebral fissure; TPS = tarsal platform show; MLD = margin-limbal distance; BUT = break-up time; TAO-QoL = thyroid associated ophthalmopathy quality of life questionnaire; LTMH = lower eyelid tear meniscus height. ∗p< 0.05, Wilcoxon signed-rank test. |
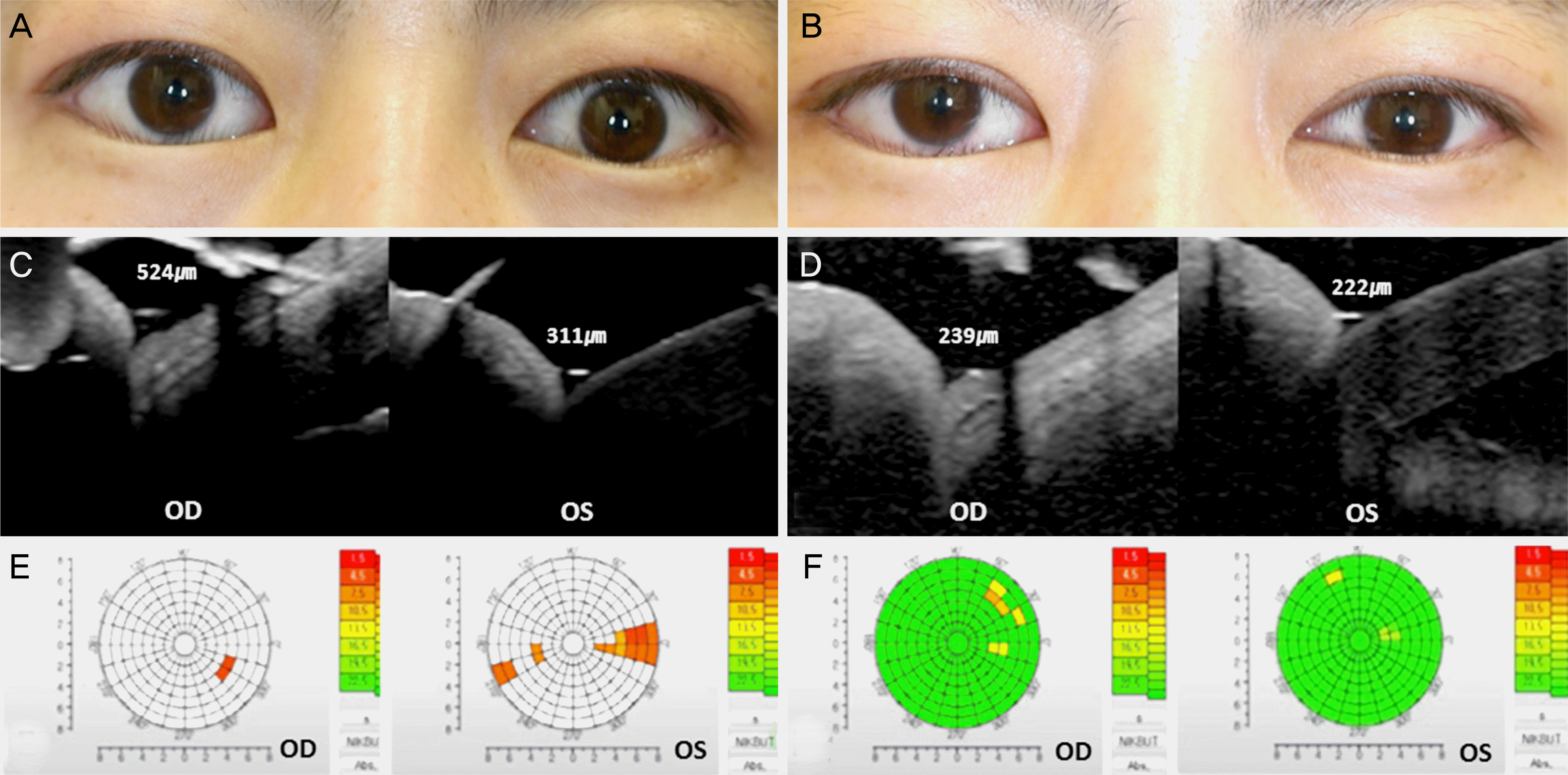 | Figure 3.Tear film stability before and after mixed injection of botulinum neurotoxin A (BoNT-A), triamcinolone and 5-fluorouracil (5-FU) in Graves’ upper eyelid retraction. 35 year-old female patient with Graves’ upper eyelid retraction (Case #9). Mixed injection of BoNT-A, triamcinolone and 5-FU was performed in the both upper eyelid. Margin reflex distance 1 (MRD1) decreased from 6.4 mm and 5.2 mm (A) to 3.8 mm and 3.9 mm (B) after 3 month. Spectralis optical coherence tomography shows lower eyelid tear meniscus height decrease after mixed injection (C, D). Keratogragh shows almost broken tear film of the left eye (E, red color) and recovered status after mixed injection (F, green color). OD = oculus dexter; OS = oculus sinister. |
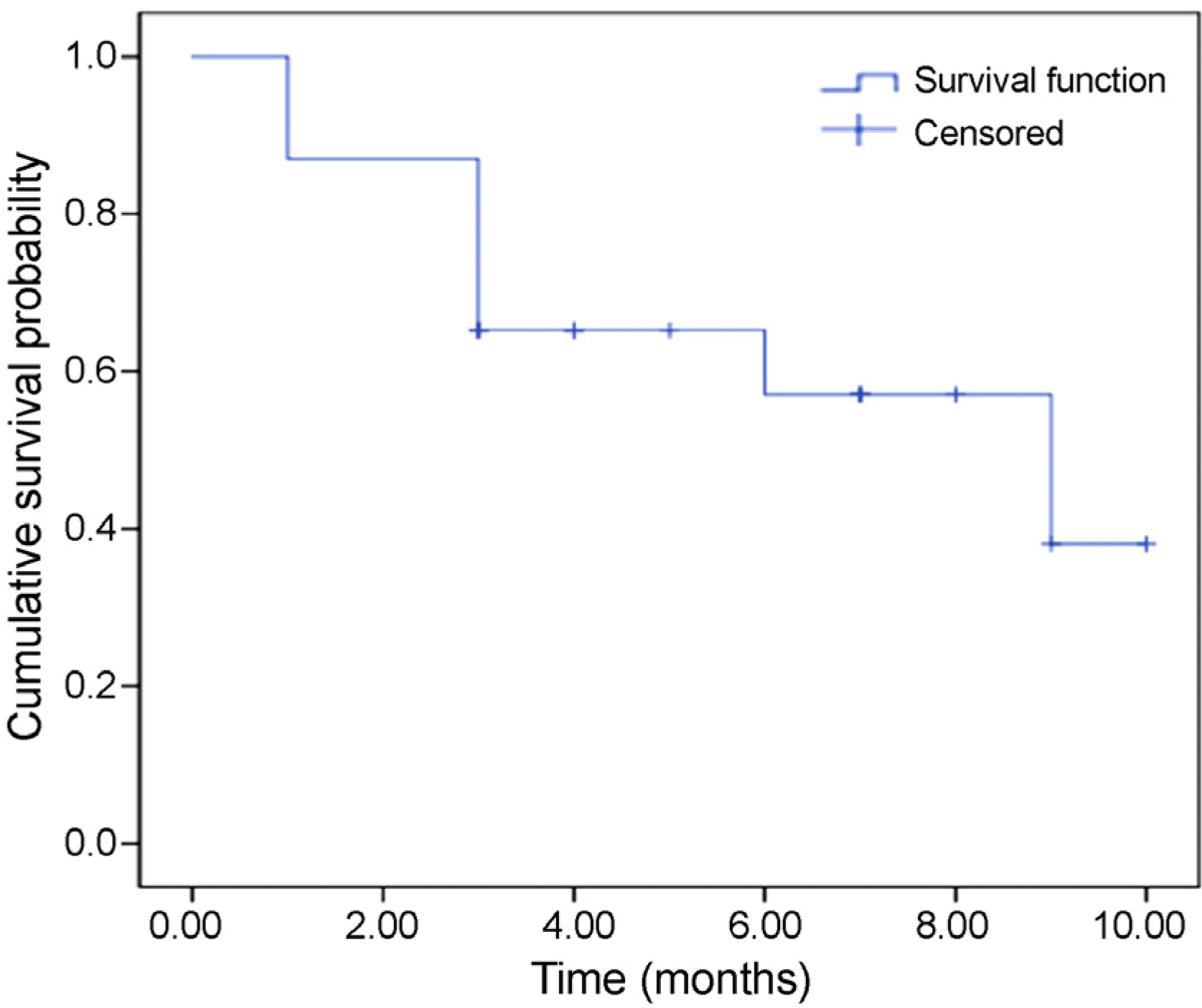 | Figure 4.Kaplan-Meier Survival Analysis for Graves’ upper eyelid retraction Correction following mixed Injection of botulinum neurotoxin A (BoNT-A), Triamcinolone and 5-fluorouracil (5-FU). |
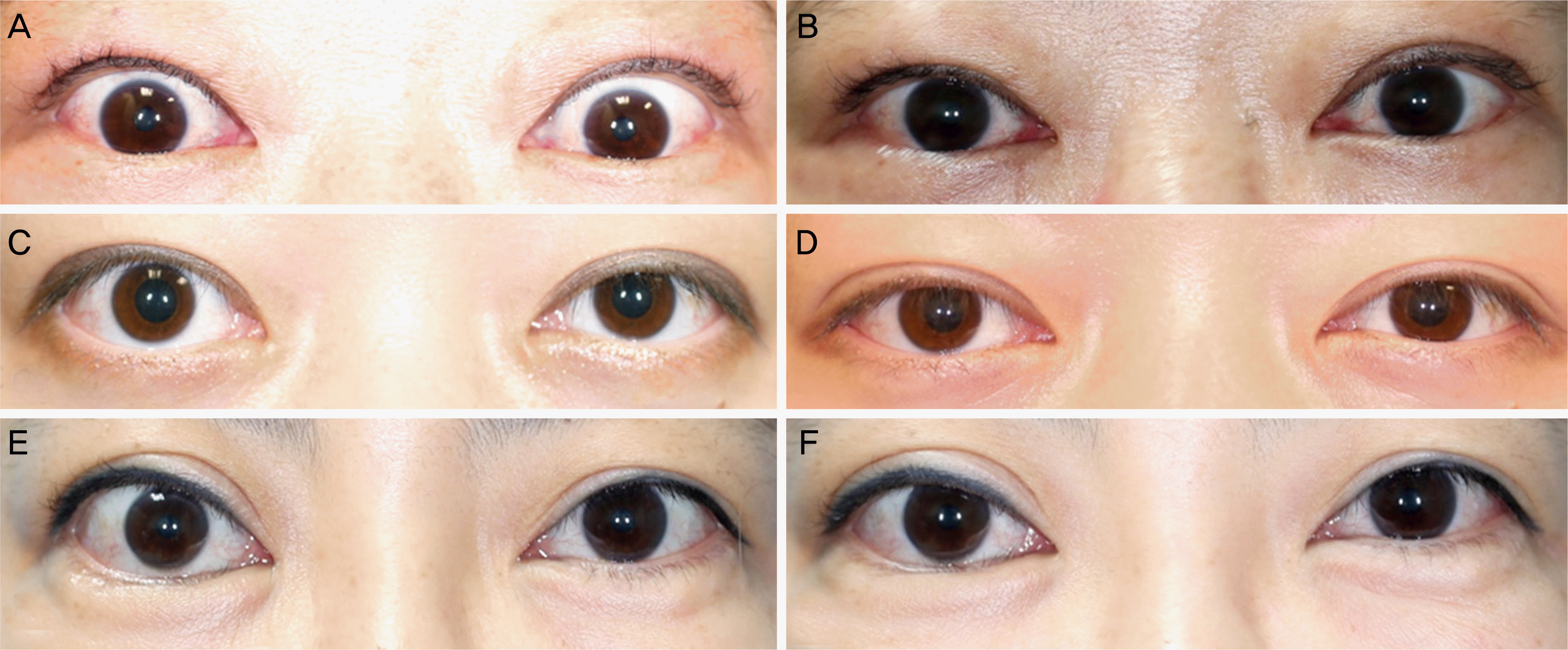 | Figure 5.Case series before and after mixed injection of botulinum neurotoxin A (BoNT-A), triamcinolone and 5-fluorouracil (5-FU) in Graves’ upper eyelid retraction. 43 year-old female patient with Graves’ upper eyelid retraction (Case #2, A, B). Mixed injection of BoNT-A, triamcinolone and 5-FU was performed in the both upper eyelid. Margin reflex distance 1 (MRD1) decreased from 6.2 mm and 6.8 mm (A) to 5.0 mm and 5.1 mm (B). 28 year-old female patient with Graves upper eyelid retraction of her right eye (Case #12, C, D). MRD1 decreased from 4.6 mm (C) to 3.4 mm (D). 40 year-old female patient with Graves upper eyelid retraction of her right eye (Case #17, E, F). MRD1 decreased from 5.2 mm (E) to 4.1 mm (F). |
Table 1.
Demographic characteristics of the patients
Table 2.
Characteristics of the Graves upper eyelid retraction patients treated with mixed injections of botulinum neurotoxin A (BoNT-A), triamcinolone and 5-fluorouracil (5-FU)
| Patient number | Sex | Age (years) | Side | Duration of Graves’ disease (months) | Follow-up (months) | CAS∗ | Thyroid status | TSHR† Ab (IU/L) | TS Ab‡ (%) | Smoking | Previous treatment |
Before injection |
One month after injection |
Three months after injection |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MRD1 (mm) | TPS (mm) | LTMH (μm) | MRD1 (mm) | TPS (mm) | LTMH (μm) | MRD1 (mm) | TPS (mm) | LTMH (μm) | ||||||||||||
| 1 | F | 34 | OD | 2 | 9 | 3 | Hypothyroid | 24.33 | 416.6 | Nonsmoker | None | 6.2 | 0.0 | 396 | 5.0 | 1.1 | 294 | 4.1 | 1.5 | 261 |
| 2 | F | 43 | OD | 2 | 4 | 6 | Hyperthyroid | 16.18 | 520.6 | Smoker | IV methylprednisolone | 6.8 | 1.5 | 141 | 5.1 | 2.2 | 374 | 4.2 | 2.0 | 152 |
| OS | 7.1 | 2.6 | 199 | 5.6 | 3.4 | 411 | 4.0 | 3.2 | 187 | |||||||||||
| 3 | M | 57 | OD | 2 | 7 | 2 | Hyperthyroid | 3.3 | 145.7 | Smoker | None | 6.8 | 0.5 | 239 | 5.4 | 0.9 | 166 | 5.5 | 0.0 | 224 |
| OS | 7.0 | 1.1 | 210 | 4.7 | 2.2 | 177 | 4.8 | 1.4 | 163 | |||||||||||
| 4 | F | 60 | OD | 3 | 17 | 1 | Hyperthyroid | >40.0 | 343.3 | Nonsmoker | None | 5.2 | 0.9 | 354 | 5.4 | 0.0 | 517 | 5.2 | 0.0 | 443 |
| OS | 5.1 | 0.0 | 355 | 5.1 | 0.0 | 690 | 4.9 | 0.0 | 470 | |||||||||||
| 5 | F | 52 | OD | 4 4 | 3 | 3 | Hyperthyroid | 4.02 | 462.6 | Nonsmoker | Oral prednisolone | 3.8 | 0.0 | 245 | 3.8 | 0.0 | 130 | 3.6 | 0.0 | 305 |
| 6 | F | 48 | OD | 4 | 3 | 4 | Hyperthyroid | 21.6 | 588.3 | Nonsmoker | None | 3.8 | 2.1 | 383 | 2.8 | 2.6 | 419 | 2.9 | 1.8 | 353 |
| OS | 4.5 | 1.0 | 285 | 3.8 | 1.2 | 321 | 3.6 | 0.7 | 293 | |||||||||||
| 7 | F | 48 | OD | 6 | 20 | 2 | Euthyroid | 3.68 | 240.1 | Nonsmoker | Oral prednisolone | 6.9 | 1.0 | 228 | 6.9 | 1.1 | 268 | 6.6 | 1.9 | 268 |
| OS | 3.5 | 3.3 | 116 | 4.0 | 2.9 | 232 | 3.3 | 3.0 | 232 | |||||||||||
| 8 | F | 27 | OS | 7 | 10 | 3 | Euthyroid | 25.65 | 809.3 | Nonsmoker | Oral prednisolone | 7.1 | 0.7 | 247 | 5.6 | 1.0 | 369 | 5.1 | 0.6 | 264 |
| 9 | F | 35 | OD | 7 | 19 | 2 | Hyperthyroid | <0.30 | 517.6 | Nonsmoker | Oral prednisolone | 6.4 | 1.5 | 524 | 3.6 | 2.2 | 239 | 3.8 | 2.3 | 222 |
| OS | 5.2 | 1.4 | 311 | 4.0 | 2.0 | 222 | 3.9 | 2.2 | 237 | |||||||||||
| 10 | F | 40 | OS | 9 | 4 | 1 | Hyperthyroid | 2.68 | 246.9 | Nonsmoker | Topical fluorometholone | 5.8 | 1.5 | 408 | 4.9 | 1.9 | 228 | 5.0 | 0.5 | 201 |
| 11 | F | 41 | OS | 9 | 9 | 2 | Hyperthyroid | 5.89 | 492.5 | Nonsmoker | Topical fluorometholone | 4.4 | 1.7 | 141 | 2.5 | 1.7 | 180 | 4.5 | 1.9 | 147 |
| 12 | F | 28 | OD | 12 | 3 | 2 | Euthyroid | 2.67 | 506.7 | Nonsmoker | Oral prednisolone Orbital decompression | 4.6 | 2.7 | 192 | 3.6 | 3.3 | 246 | 3.4 | 3.4 | 181 |
| 13 | F | 58 | OS | 18 | 6 | 2 | Hyperthyroid | 2.49 | 162.5 | Nonsmoker | None | 3.7 | 0.0 | 207 | 6.0 | 0.0 | 233 | 5.3 | 0.0 | 222 |
| 14 | F | 52 | OS | 30 | 11 | 2 | Hyperthyroid | >40.0 | 633.9 | Nonsmoker | Oral prednisolone | 5.7 | 3.3 | 387 | 5.1 | 3.2 | 217 | 4.9 | 4.3 | 264 |
| 15 | F | 51 | OS | 24 | 18 | 3 | Hyperthyroid | >40.0 | 488.1 | Nonsmoker | None | 5.6 | 4.3 | 607 | 5.4 | 3.9 | 387 | 4.7 | 4.4 | 356 |
| 16 | F | 32 | OD | 36 | 7 | 2 | Hyperthyroid | 6.48 | 493.2 | Nonsmoker | None | 6.7 | 0.0 | 117 | 6.1 | 0.7 | 152 | 6.6 | 0.0 | 130 |
| OS | 7.3 | 0.0 | 90 | 5.4 | 0.7 | 130 | 6.1 | 0.0 | 115 | |||||||||||
|
17 |
F |
40 |
OD |
120 |
3 |
4 |
Hyperthyroid |
>40.0 |
517.1 |
Smoker |
Oral prednisolone |
5.2 |
3.6 |
278 |
4.1 |
4.5 |
470 |
4.1 |
4.0 |
275 |
|
Mean |
|
43.9 |
|
17.4 |
9.0 |
2.6 |
|
446.2 |
|
5.6 |
1.4 |
277.5 |
4.7 |
1.8 |
294.7 |
4.6 |
1.6 |
248.5 |
||
| SD | 10.4 | 28.3 | 6.0 | 1.2 | 172.7 | 1.2 | 1.3 | 130.2 | 1.1 | 1.3 | 137.4 | 1.0 | 1.5 | 90.4 | ||||||
Table 3.
Clinical outcomes after mixed injections of botulinum neurotoxin A (BoNT-A), triamcinolone and 5-fluorouracil (5-FU)
| Parameters | Before injection | 1 month after injection | 3 months after injection | Last follow-up | |
|---|---|---|---|---|---|
| Eyelid position | MRD1 | 5.6 ± 1.2 | 4.7 ± 1.1∗ | 4.6 ± 1.0∗ | 5.2 ± 0.9 |
| MRD2 | 5.1 ± 0.9 | 5.2 ± 0.9 | 5.0 ± 1.0 | 5.1 ± 0.9 | |
| IPF | 10.6 ± 1.4 | 10.0 ± 1.4 | 9.8 ± 1.4∗ | 10.3 ± 1.2 | |
| TPS | 1.4 ± 1.3 | 1.8 ± 1.3∗ | 1.6 ± 1.5 | 1.7 ± 1.4 | |
| MLD | 10.3 ± 0.9 | 9.8 ± 0.9 | 10.0 ± 1.1 | 10.0 ± 0.7 | |
| Tear film stability | LTMH | 277.5 ± 130.2 | 294.7.1 ± 137.4 | 248.5.0 ± 90.4† | 297.0 ± 138.1 |
| BUT | 5.2 ± 3.1 | 10.3 ± 7.8∗ | - | - | |
| TAO-QoL | Sum of scores | 12.4 ± 6.6 | - | - | 10.1 ± 8.6∗ |
Table 4.
Kaplan-Meier survival analysis and success rate for upper eyelid retraction correction following mixed injections of botulinum neurotoxin A (BoNT-A), triamcinolone and 5-fluorouracil (5-FU)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download