Abstract
Purpose
To report two cases of phacoemulsification and intraocular lens implantation after inadvertent intralenticular injection of a dexamethasone implant.
Case summary
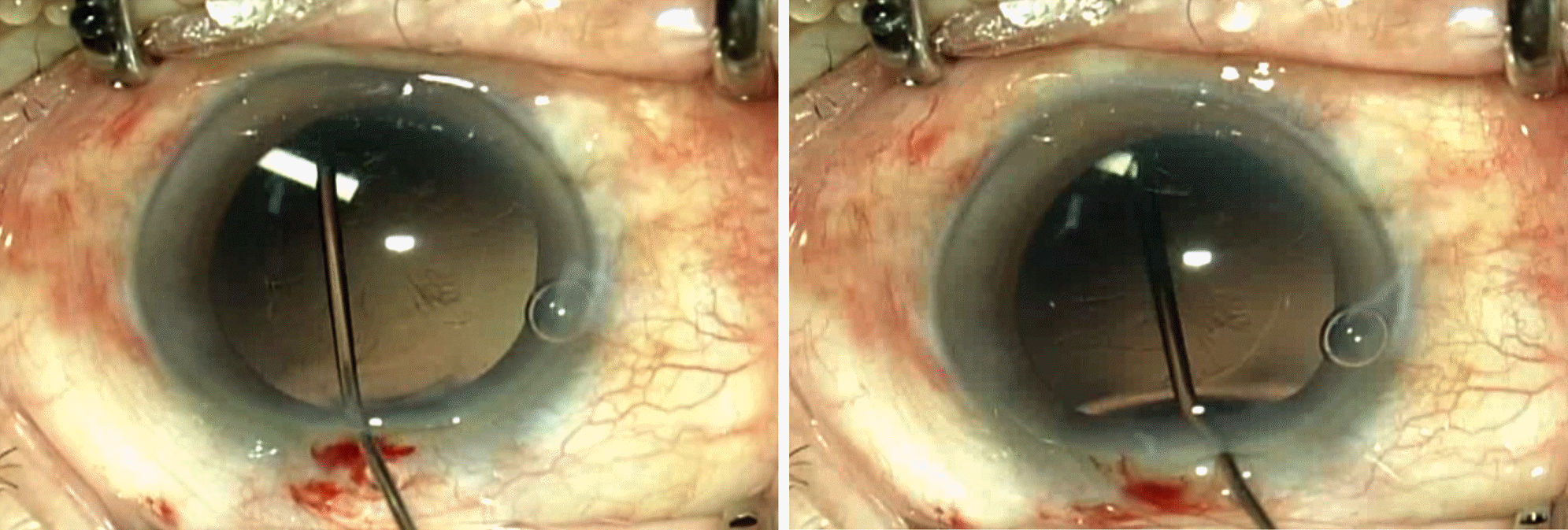
(Case 1) A 73-year-old male was referred to our hospital after an accidental intralenticular injection of a dexamethasone implant in a local clinic for treatment of branch retinal vein occlusion in his right eye. During the follow-up period, posterior capsular opacity progressed and phacoemulsification and intraocular lens implantation were performed 10 days later. During the surgery, the dexamethasone implant shattered and could not be repositioned into the vitreous cavity. The remnants of that implant were removed and a second dexamethasone implant was successfully injected into the vitreous cavity. (Case 2) A 69-year-old female was being treated for branch retinal vein occlusion in her left eye in our hospital. A dexamethasone implant was accidentally injected into her lens, thus phacoemulsification and intraocular lens implantation were performed the following day. During the procedure, we were able to successfully reposition the dexamethasone implant into the vitreous cavity.
Conclusions
Cataract formation after intralenticular injection of a dexamethasone implant can be easily managed with phacoemulsification. However, the dexamethasone implant shattered 10 days after the injection and could not be repositioned. The implant kept its hardness for at least one day and we were able to reposition it into the vitreous cavity without extending the rupture site of the posterior capsule.
Go to : 
References
1. Gan IM, Ugahary LC, van Dissel JT, van Meurs JC. Effect of intravitreal dexamethasone on vitreous vancomycin concentrations in patients with suspected postoperative bacterial endophthalmitis. Graefes Arch Clin Exp Ophthalmol. 2005; 243:1186–9.

2. Haller JA, Bandello F, Belfort R Jr, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011; 118:2453–60.
3. Cavalcante LL, Cavalcante ML, Murray TG, et al. Intravitreal injection analysis at the Bascom Palmer Eye Institute: evaluation of clinical indications for the treatment and incidence rates of endophthalmitis. Clin Ophthalmol. 2010; 4:519–24.

4. Coca-Robinot J, Casco-Silva B, Armadá-Maresca F, García-Martínez J. Accidental injections of dexamethasone intravitreal implant (Ozurdex) into the crystalline lens. Eur J Ophthalmol. 2014; 24:633–6.

5. Meyer CH, Rodrigues EB, Michels S, et al. Incidence of damage to the crystalline lens during intravitreal injections. J Ocul Pharmacol Ther. 2010; 26:491–5.

6. Koller S, Neuhann T, Neuhann I. Conspicuous crystalline lens foreign body after intravitreal injection. Ophthalmologe. 2012; 109:1119–21.
7. Karalezli A, Eroglu FC. Intravitreal dexamethasone implant in the crystalline lens. JCRS Online Case Reports. 2014; 2:e12–5.

8. Hattenbach LO, Klais C, Koch FH, Gümbel HO. Intravitreous injection of tissue plasminogen activator and gas in treatment of submacular hemorrhage under various conditions. Ophthalmology. 2001; 108:1485–92.
Go to : 
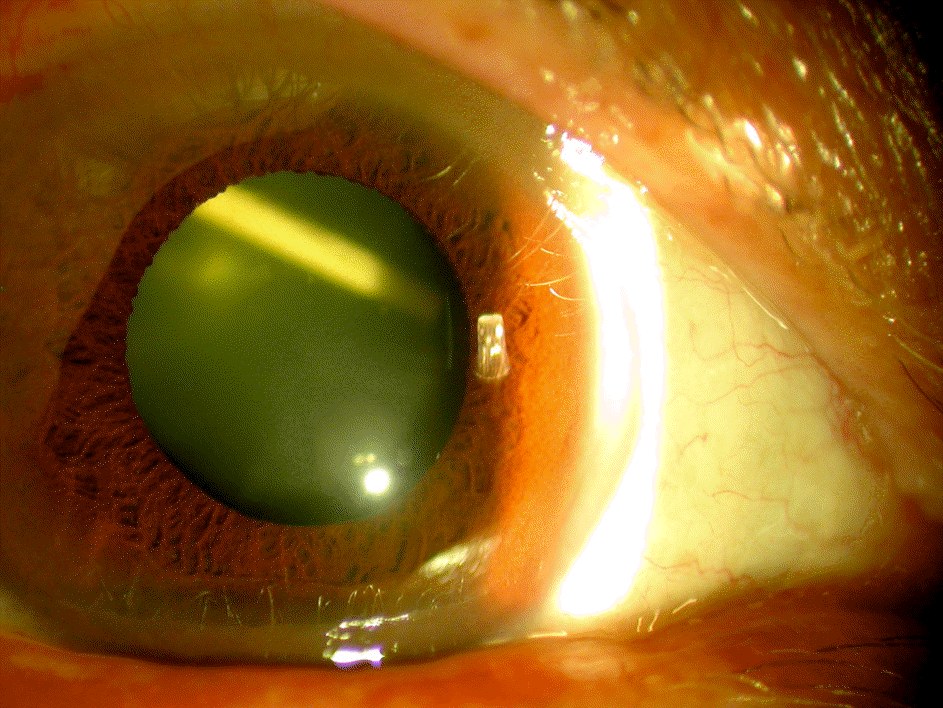 | Figure 1.Photograph showing inadvertent intralenticular injection of dexamethasone implant. Dexamethasone implant was inadvertently injected into the lens. |
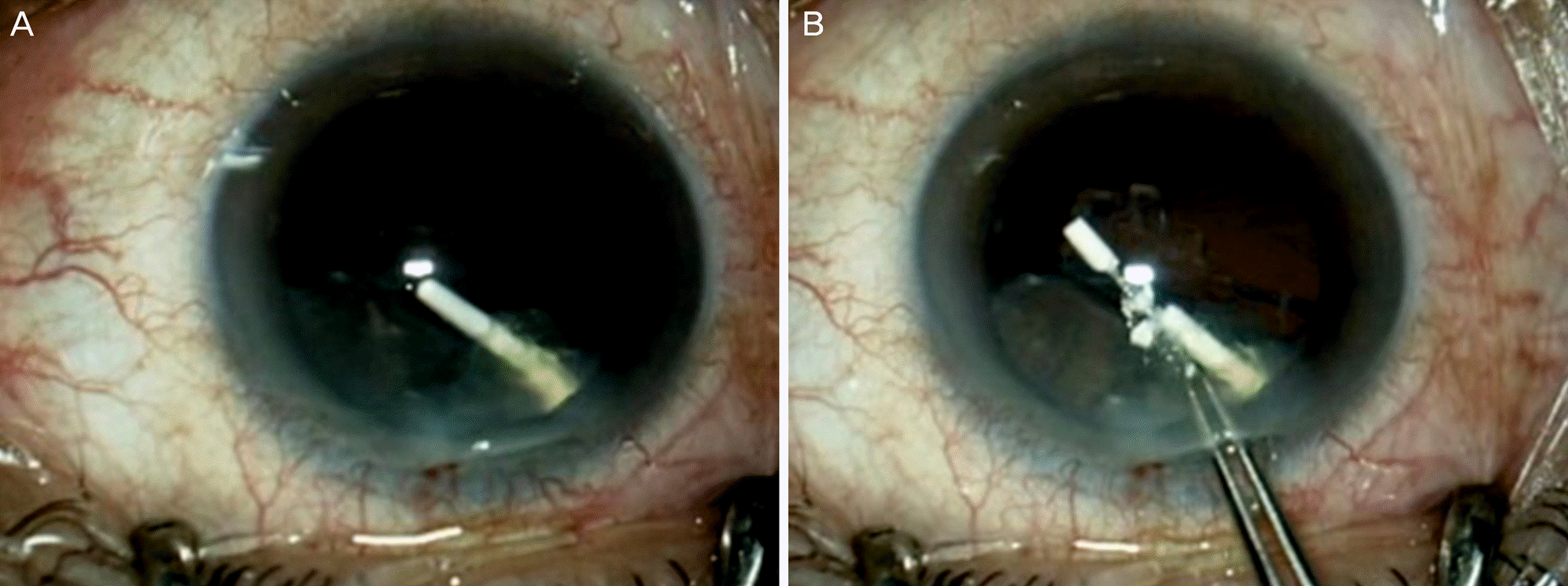 | Figure 2.Intraoperative photograph. (A) Intraoperative photograph showing the dexamethasone implant located in the lens. (B) The dexamethasone implant shattered when retrieved with forceps. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download