Abstract
Purpose
To compare the aqueous concentrations of cytokines in glaucomatous eyes with those of normal controls and to characterize the clinical factors associated with aqueous cytokine concentration.
Methods
In this cross-sectional study, aqueous humor samples were collected from 54 eyes of 54 patients (34 medically treated primary open angle glaucoma and 20 normal controls) during cataract surgery from January 2014 to January 2015. Glaucoma patients were divided into two groups: patients using prostaglandin analogue for more than 6 months (prostaglandin F2α analogue [PGA] user) and patients with no experience of PGA use (PGA non-user). The levels of cytokines (matrix metalloproteinase [MMP]1, MMP9, MMP3, vascular endothelial growth factor, interleukin [IL]-1, IL-8, tumor necrosis factor [TNF]-α) in the aqueous of glaucoma and control subjects were quantified using a multiplex cytokine analysis.
Results
Aqueous humor collected from the glaucoma patients exhibited significantly increased concentrations of MMP1 (p = 0.002) and MMP9 (p = 0.026). Among glaucoma patients, PGA users showed significantly higher level of MMP 9 compared with PGA non-users (p = 0.003). In the univariate and multivariate linear regression analyses, PGA use (ß = 0.351, p = 0.027) and vertical cup-to disc ratio (ß = −0.401, p = 0.013) were the significant risk factors associated with the level of MMP9.
Go to : 
References
1. Tamm ER. The trabecular meshwork outflow pathways: structural and functional aspects. Exp Eye Res. 2009; 88:648–55.

2. Markiewicz L, Pytel D, Mucha B, et al. Altered expression levels of MMP1, MMP9, MMP12, TIMP1, and IL-1β as a risk factor for the elevated IOP and optic nerve head damage in the primary open-angle glaucoma patients. Biomed Res Int. 2015; 2015. 812503.
3. Weinstein WL, Dietrich UM, Sapienza JS, et al. Identification of ocular matrix metalloproteinases present within the aqueous humor and iridocorneal drainage angle tissue of normal and glaucomatous canine eyes. Vet Ophthalmol. 2007; 10(Suppl 1):108–16.

4. Chua J, Vania M, Cheung CM, et al. Expression profile of inflammatory cytokines in aqueous from glaucomatous eyes. Mol Vis. 2012; 18:431–8.
5. Pradhan ZS, Dalvi RA, Lai T, et al. Prostaglandin agonist effect on matrix metalloproteinase aqueous levels in glaucoma patients. Can J Ophthalmol. 2015; 50:6–10.

6. Chintala SK, Wang N, Diskin S, et al. Matrix metalloproteinase gelatinase B (MMP-9) is associated with leaking glaucoma filtering blebs. Exp Eye Res. 2005; 81:429–36.

7. duPont NC, Wang K, Wadhwa PD, et al. Validation and comparison of luminex multiplex cytokine analysis kits with ELISA: determinations of a panel of nine cytokines in clinical sample culture supernatants. J Reprod Immunol. 2005; 66:175–91.

8. Alexander JP, Samples JR, Van Buskirk EM, Acott TS. Expression of matrix metalloproteinases and inhibitor by human trabecular meshwork. Invest Ophthalmol Vis Sci. 1991; 32:172–80.
9. De Groef L, Van Hove I, Dekeyster E, et al. MMPs in the neuroretina and optic nerve: modulators of glaucoma pathogenesis and repair? Invest Ophthalmol Vis Sci. 2014; 55:1953–64.

10. Nakakura S, Tabuchi H, Baba Y, et al. Comparison of the latanoprost 0. 005%/timolol 0.5% + brinzolamide 1% versus dorzolamide 1%/timolol 0.5% + latanoprost 0.005%: a 12-week, randomized open-label trial. Clin Ophthalmol. 2012; 6:369–75.
11. Toris CB, Gabelt BT, Kaufman PL. Update on the mechanism of action of topical prostaglandins for intraocular pressure reduction. Surv Ophthalmol. 2008; 53(Suppl 1):S107–20.

12. Weinreb RN, Kashiwagi K, Kashiwagi F, et al. Prostaglandins increase matrix metalloproteinase release from human ciliary smooth muscle cells. Invest Ophthalmol Vis Sci. 1997; 38:2772–80.
13. Weinreb RN, Lindsey JD, Marchenko G, et al. Prostaglandin FP agonists alter metalloproteinase gene expression in sclera. Invest Ophthalmol Vis Sci. 2004; 45:4368–77.

14. Kim JW, Lindsey JD, Wang N, Weinreb RN. Increased human scleral permeability with prostaglandin exposure. Invest Ophthalmol Vis Sci. 2001; 42:1514–21.
15. Oh DJ, Martin JL, Williams AJ, et al. Effect of latanoprost on the expression of matrix metalloproteinases and their tissue inhibitors in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2006; 47:3887–95.

16. Lopilly Park HY, Kim JH, Lee KM, Park CK. Effect of prostaglandin analogues on tear proteomics and expression of cytokines and matrix metalloproteinases in the conjunctiva and cornea. Exp Eye Res. 2012; 94:13–21.

17. Jia Y, Hu DN, Zhu D, et al. MMP-2, MMP-3, TIMP-1, TIMP-2, and TIMP-3 protein levels in human aqueous humor: relationship with axial length. Invest Ophthalmol Vis Sci. 2014; 55:3922–8.

18. Mohammad G, Kowluru RA. Novel role of mitochondrial matrix metalloproteinase-2 in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011; 52:3832–41.

19. Mohammad G, Kowluru RA. Matrix metalloproteinase-2 in the development of diabetic retinopathy and mitochondrial dysfunction. Lab Invest. 2010; 90:1365–72.

20. Hoffmann S, He S, Ehren M, et al. MMP-2 and MMP-9 secretion by rpe is stimulated by angiogenic molecules found in choroidal neovascular membranes. Retina. 2006; 26:454–61.

21. Das A, McGuire PG, Eriqat C, et al. Human diabetic neovascular membranes contain high levels of urokinase and metalloproteinase enzymes. Invest Ophthalmol Vis Sci. 1999; 40:809–13.
22. Hu J, Van den Steen PE, Sang QX, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007; 6:480–98.

23. Kawasaki Y, Xu ZZ, Wang X, et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008; 14:331–6.

Go to : 
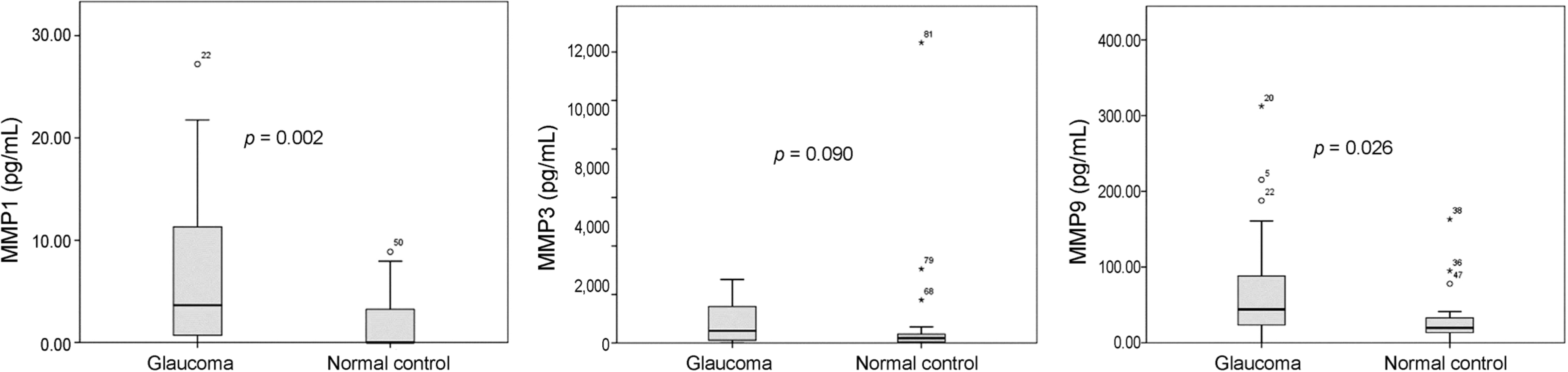 | Figure 1.Comparisons of the aqueous concentrations of matrix metalloproteinase 1, 3, and 9 between glaucoma patients and normal controls. All the comparisons were performed by independent t-test. MMP = matrix metalloproteinase. |
Table 1.
Clinical characteristics of glaucoma patients and normal controls
Table 2.
Comparison of clinical characteristics and aqueous cytokine concentrations between glaucoma patients using prostaglandin analogues for longer than 6 months and patients with no experience of prostaglandin analogues
Table 3.
Clinical factors affecting aqueous concentration of MMP9 in glaucoma patients




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download