Abstract
Purpose
To evaluate the influence of retinal collateral vessels in eyes with macular edema secondary to branch retinal vein occlusion (BRVO) treated with intravitreal bevacizumab (IVB).
Methods
We reviewed the medical records of patients with BRVO who were followed up for 12 months. To compare formations of collateral vessels, the patients were divided into 2 groups. The treatment group included 20 patients (20 eyes) treated with IVB, and the control group included 41 patients (41 eyes) without treatment.
Results
In the treatment group, the mean age was 58.4 ± 9.5 years. The average number of IVB injections performed during the 12 months was 4.5 ± 2.5 (range 2 to 8). After 12 months from diagnosis, 13 eyes (65%) presented with collateral vessels. In the control group, the mean age was 60.6 ± 9.3 years and 28 eyes (68.3%) presented with collateral vessel after 12 months. There was no difference in incidence of collateral vessel formation between the treatment group and the control group (p = 0.574).
References
1. Argon laser photocoagulation for macular edema in branch vein occlusion. The Branch Vein Occlusion Study Group. Am J Ophthalmol. 1984; 98:271–82.
2. Klein R, Moss SE, Meuer SM, Klein BE. The 15-year cumulative incidence of retinal vein occlusion: the Beaver Dam Eye Study. Arch Ophthalmol. 2008; 126:513–8.
3. Avitabile T, Longo A, Reibaldi A. Intravitreal triamcinolone compared with macular laser grid photocoagulation for the treatment of cystoid macular edema. Am J Ophthalmol. 2005; 140:695–702.

4. Ehlers JP, Decroos FC, Fekrat S. Intravitreal bevacizumab for macular edema secondary to branch retinal vein occlusion. Retina. 2011; 31:1856–62.

5. Lee YS, Kim MS, Yu SY, Kwak HW. Two-year results of intravitreal bevacizumab injection in retinal vein occlusion. J Korean Ophthalmol Soc. 2011; 52:1039–47.

6. Rabena MD, Pieramici DJ, Castellarin AA, et al. Intravitreal bevacizumab (Avastin) in the treatment of macular edema secondary to branch retinal vein occlusion. Retina. 2007; 27:419–25.

7. Russo V, Barone A, Conte E, et al. Bevacizumab compared with macular laser grid photocoagulation for cystoid macular edema in branch retinal vein occlusion. Retina. 2009; 29:511–5.

8. Koss MJ, Pfister M, Rothweiler F, et al. Comparison of cytokine levels from undiluted vitreous of untreated patients with retinal vein occlusion. Acta Ophthalmol. 2012; 90:e98–103.

9. Campochiaro PA, Bhisitkul RB, Shapiro H, Rubio RG. Vascular endothelial growth factor promotes progressive retinal non-perfusion in patients with retinal vein occlusion. Ophthalmology. 2013; 120:795–802.

10. Kim KS, Chang HR, Song S. Ischaemic change after intravitreal bevacizumab (Avastin) injection for macular oedema secondary to non-ischaemic central retinal vein occlusion. Acta Ophthalmol. 2008; 86:925–7.

11. Sabet-Peyman EJ, Heussen FM, Thorne JE, et al. Progression of macular ischemia following intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging. 2009; 40:316–8.

12. Goldberg N, Freund KB. Progressive optic nerve collateralization after serial intravitreal ranibizumab injections for central retinal vein occlusion. Retina. 2013; 33:449–50.

13. Seo S, Yang Y. Formation of collateral vessels and new vessels in branch retinal vein occlusion. J Korean Ophthalmol Soc. 2009; 50:61–8.

14. Im CY, Lee SY, Kwon OW. Collateral vessels in branch retinal vein occlusion. Korean J Ophthalmol. 2002; 16:82–7.

15. Montero-Moreno JA, Ruiz-Moreno JM. Collateral vessels after retinal vein occlusion treated with intravitreal bevacizumab. Austin J Clin Ophthalmol. 2014; 1:1028.
Figure 1.
Changes in BCVA and CMT from baseline to 12 months after treatment in treatment group. BCVA = best corrected visual acuity; CMT = central macular thickness.
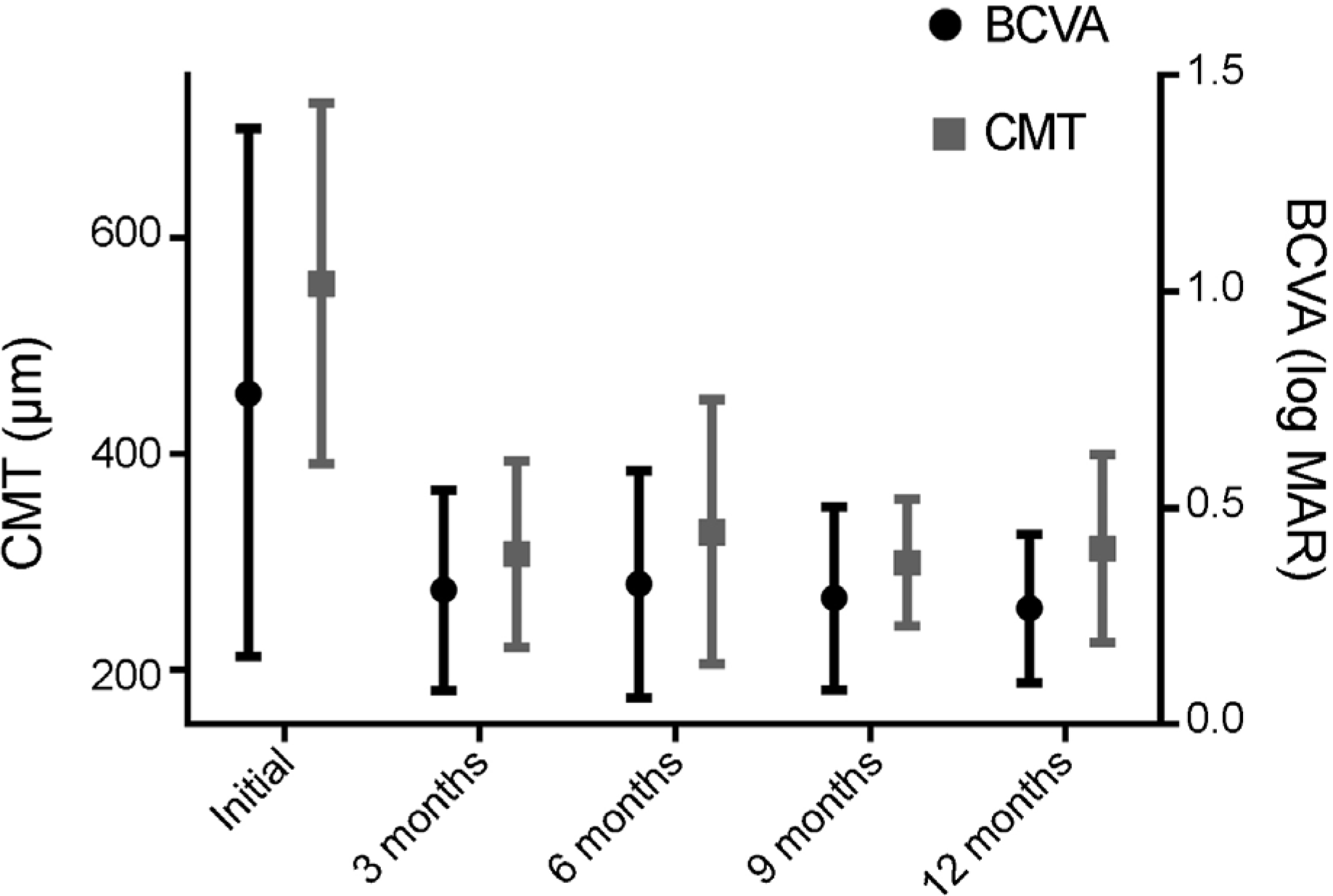
Figure 2.
Collateral vessel in branch retinal vein occlusion (white arrows; A : on the optic disc, B: around occlusion site, C: across horizontal raphe).

Table 1.
Characteristics of the patients
| Variable | Treatment group (20 eyes) | Control group (41 eyes) | p-value |
|---|---|---|---|
| Sex (%) | 0.287* | ||
| Male | 7 (35.0) | 19 (46.3) | |
| Female | 13 (65.0) | 22 (53.7) | |
| Age (years) | 0.460† | ||
| Mean ± SD | 58.4 ± 9.5 | 60.6 ± 9.3 | |
| Range | 41-73 | 41-85 | |
| Underline disease (%) | 0.488* | ||
| DM | 2 (10) | 4 (9.8) | |
| HTN | 6 (30) | 16 (39.0) | |
| Both (DM and HTN) | 10 (50) | 4 (9.8) | |
| Laterality (eyes, %) | 0.430* | ||
| Right | 10 (50) | 18 (43.9) | |
| Left | 10 (50) | 23 (56.1) | |
| Involved branch (eyes, %) | 0.613* | ||
| ST | 9 (45) | 21 (51.2) | |
| IT | 6 (30) | 14 (34.1) | |
| Macular | 5 (25) | 6 (14.6) | |
| Collateral vessel formation (eyes, %) | 0.574* | ||
| Yes | 13 (65) | 28 (68.3) | |
| No | 7 (35) | 13 (31.7) |
Table 2.
Comparison of collateral vessel formation group and no collateral vessel formation group
| Collateral vessel formation | No collateral vessel formation | p-value* | |
|---|---|---|---|
| Treatment group | |||
| No. of patients | 13 | 7 | |
| BCVA (log MAR) | |||
| Initial | 0.67 ± 0.33 | 0.93 ± 0.94 | 0.968 |
| At 12 months | 0.25 ± 0.17 | 0.29 ± 0.18 | 0.628 |
| CMT (μm) | |||
| Initial | 552.0 ± 164.5 | 568.8 ± 181.5 | 0.843 |
| At 12 months | 314.5 ± 106.5 | 309.5 ± 70.8 | 0.451 |
| Number of IVB injection | |||
| Mean | 4.7 | 4.3 | 0.384 |
| Range | 2-7 | 2-8 | |
| Control group | |||
| No. of patients | 28 | 13 | |
| BCVA (log MAR) | |||
| Initial | 0.69 ± 0.49 | 0.64 ± 0.27 | 0.573 |
| At 12 months | 0.19 ± 0.10 | 0.24 ± 0.11 | 0.040 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download