Abstract
Purpose
To evaluate the effects of continuous curvilinear capsulorhexis, intraocular lens (IOL) decentration and tilt on postoperative clinical outcomes after cataract surgery.
Methods
We reviewed 62 eyes of 52 patients who underwent cataract surgery and measured the uncorrected visual acuity, best corrected visual acuity and manifest refraction preoperatively and 3 months postoperatively. IOL decentration on anterior segment photography and IOL tilt on anterior optical coherent tomography were analyzed and correlations of postoperative uncorrected visual acuity, best corrected visual acuity, and higher order aberrations were evaluated. In addition, we inspected the relationship of size and decentration of continuous curvilinear capsulorhexis (CCC) intraoperatively with the change in IOL position postoperatively.
Results
The average size of CCC was 5.40 ± 0.51 mm (4.12-6.24 mm) and the average decentration of CCC was 0.30 ± 0.19 mm (0.09-1.21 mm) intraoperatively. The average decentration of IOL was 0.23 ± 0.15 mm (0.00-0.71 mm) and the average IOL tilt was 1.43 ± 0.73° (0.00-4.22°) postoperatively. Intraoperative CCC size and decentration were associated with postoperative IOL decentration (p = 0.01, p < 0.001), but not with IOL tilt (p = 0.69, p = 0.52). There were no significant correlations between IOL decentration and tilt with postoperative visual outcomes and higher order aberrations.
Go to : 
References
1. Hayashi K, Hayashi H, Nakao F, Hayashi F. Anterior capsule contraction and intraocular lens decentration and tilt after hydrogel lens implantation. Br J Ophthalmol. 2001; 85:1294–7.

2. Baumeister M, Bühren J, Kohnen T. Tilt and decentration of spherical and aspheric intraocular lenses: effect on higher-order aberrations. J Cataract Refract Surg. 2009; 35:1006–12.

3. Korynta J, Bok J, Cendelin J, Michalova K. Computer modeling of visual impairment caused by intraocular lens misalignment. J Cataract Refract Surg. 1999; 25:100–5.

4. Kránitz K, Miháltz K, Sándor GL, et al. Intraocular lens tilt and decentration measured by Scheimpflug camera following manual or femtosecond laser-created continuous circular capsulotomy. J Refract Surg. 2012; 28:259–63.

5. Kránitz K, Takacs A, Miháltz K, et al. Femtosecond laser capsulotomy and manual continuous curvilinear capsulorrhexis parameters and their effects on intraocular lens centration. J Refract Surg. 2011; 27:558–63.

6. Lee WS, Han SY, Lee KH. Comparison of Laser Refractive Cataract Surgery with a Femtosecond Laser Versus Conventional Phacoemulsification. J Korean Ophthalmol Soc. 2013; 54:1127–35.

7. Seo SJ, Lee DH, Joo CK. The effect of a capsular tension ring on decentration and tilting of intraocular lenses after cataract surgery. J Korean Ophthalmol Soc. 2002; 43:29–34.
8. Cha YD, Oh SH, Lee DH. Comparative assessment of clinical results in various acrylate IOLs. J Korean Ophthalmol Soc. 2006; 47:740–7.
9. Choi YJ, Chung SK. Comparison of decentration and tilt in foldable acrylic intraocular lenses. J Korean Ophthalmol Soc. 2006; 47:37–41.
10. Lee JY, Lee SH, Chung SK. Decentration, tilt and anterior chamber depth: aspheric vs spheric acrylic intraocular lens. J Korean Ophthalmol Soc. 2009; 50:852–7.

11. de Castro A, Rosales P, Marcos S. Tilt and decentration of intraocular lenses in vivo from Purkinje and Scheimpflug imaging. Validation study. J Cataract Refract Surg. 2007; 33:418–29.
12. Kumar DA, Agarwal A, Prakash G, et al. Evaluation of intraocular lens tilt with anterior segment optical coherence tomography. Am J Ophthalmol. 2011; 151:406–12.e2.

13. Zhang Q, Jin W, Wang Q. Repeatability, reproducibility, and agreement of central anterior chamber depth measurements in pseudophakic and phakic eyes: optical coherence tomography versus ultrasound biomicroscopy. J Cataract Refract Surg. 2010; 36:941–6.

14. Mura JJ, Pavlin CJ, Condon GP, et al. Ultrasound biomicroscopic analysis of iris-sutured foldable posterior chamber intraocular lenses. Am J Ophthalmol. 2010; 149:245–52.e2.

15. Li L, Wang K, Yan Y, et al. Research on calculation of the IOL tilt and decentration based on surface fitting. Comput Math Methods Med. 2013; 2013:572530.

16. Ohmi S. Decentration associated with asymmetric capsular shrinkage and intraocular lens size. J Cataract Refract Surg. 1993; 19:640–3.

17. Gimbel HV, Neuhann T. Development, advantages, and methods of the continuous circular capsulorhexis technique. J Cataract Refract Surg. 1990; 16:31–7.

18. Friedman NJ, Palanker DV, Schuele G, et al. Femtosecond laser capsulotomy. J Cataract Refract Surg. 2011; 37:1189–98.

19. Okada M, Hersh D, Paul E, van der Straaten D. Effect of centration and circularity of manual capsulorrhexis on cataract surgery refractive outcomes. Ophthalmology. 2014; 121:763–70.

20. Masket S. Postoperative complications of capsulorhexis. J Cataract Refract Surg. 1993; 19:721–4.

21. Ravalico G, Tognetto D, Palomba M, et al. Capsulorhexis size and posterior capsule opacification. J Cataract Refract Surg. 1996; 22:98–103.

22. Hollick EJ, Spalton DJ, Meacock WR. The effect of capsulorhexis size on posterior capsular opacification: one-year results of a randomized prospective trial. Am J Ophthalmol. 1999; 128:271–9.

23. Miháltz K, Knorz MC, Alió JL, et al. Internal aberrations and optical quality after femtosecond laser anterior capsulotomy in cataract surgery. J Refract Surg. 2011; 27:711–6.

Go to : 
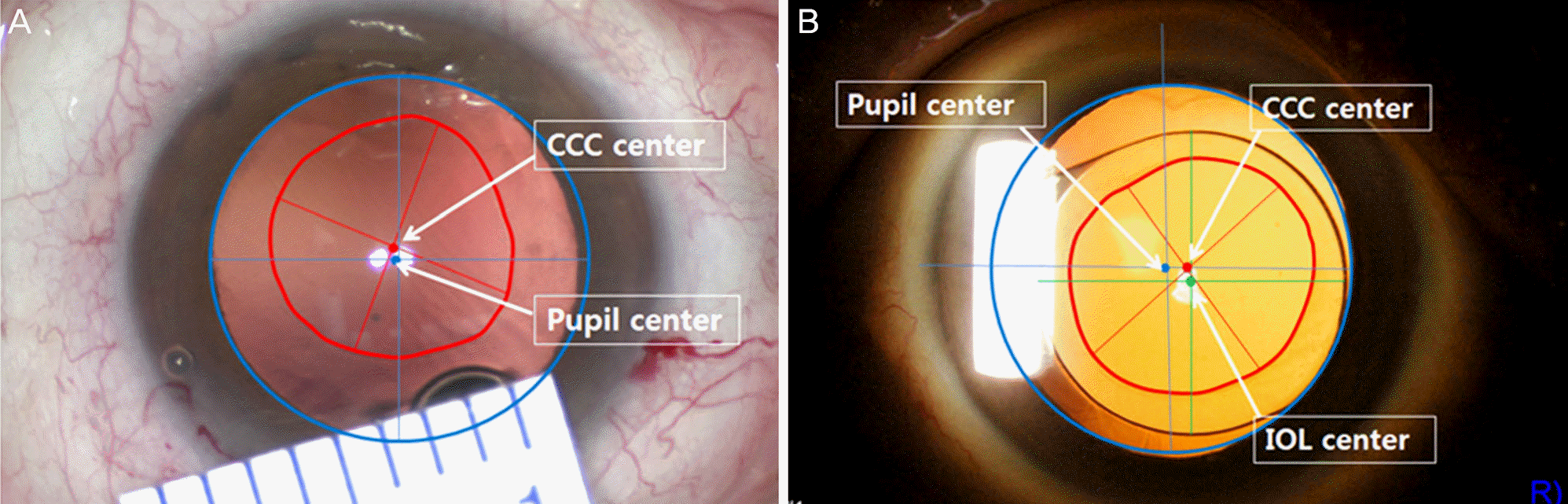 | Figure 1.Anterior segment photography. Measurements of CCC size and decentration from the dilated pupil center in the intraoperative state (A) and IOL decantation in the postoperative state (B). CCC = continuous circular capsulorhexis; IOL = intraocular lens. |
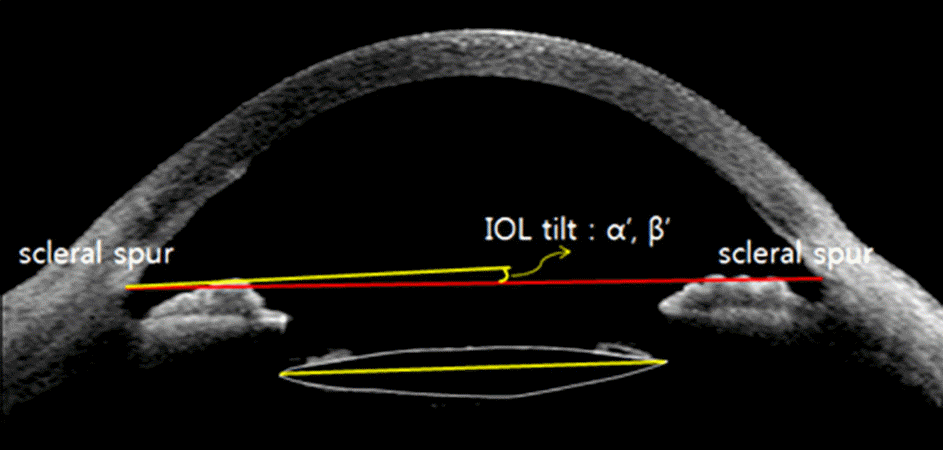 | Figure 2.Measurement of IOL tilt angle in the x-axis (α‘) and y-axis (β‘) from the line connecting both scleral spurs using anterior segment OCT (Visante™ OCT). IOL = intraocular lens; OCT = optical coherence tomography. |
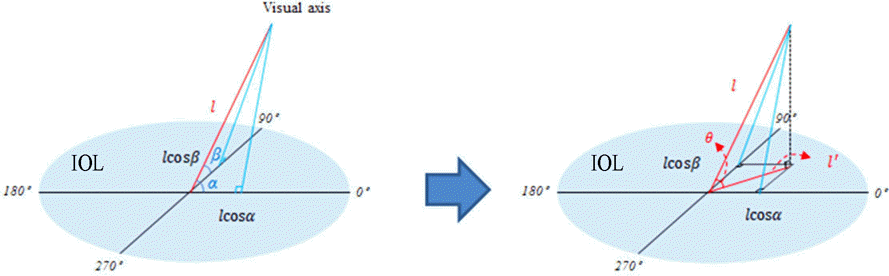 | Figure 3.Schematic images of calculation of intraocular lens tilt direction from visual axis. IOL = intraocular lens. |
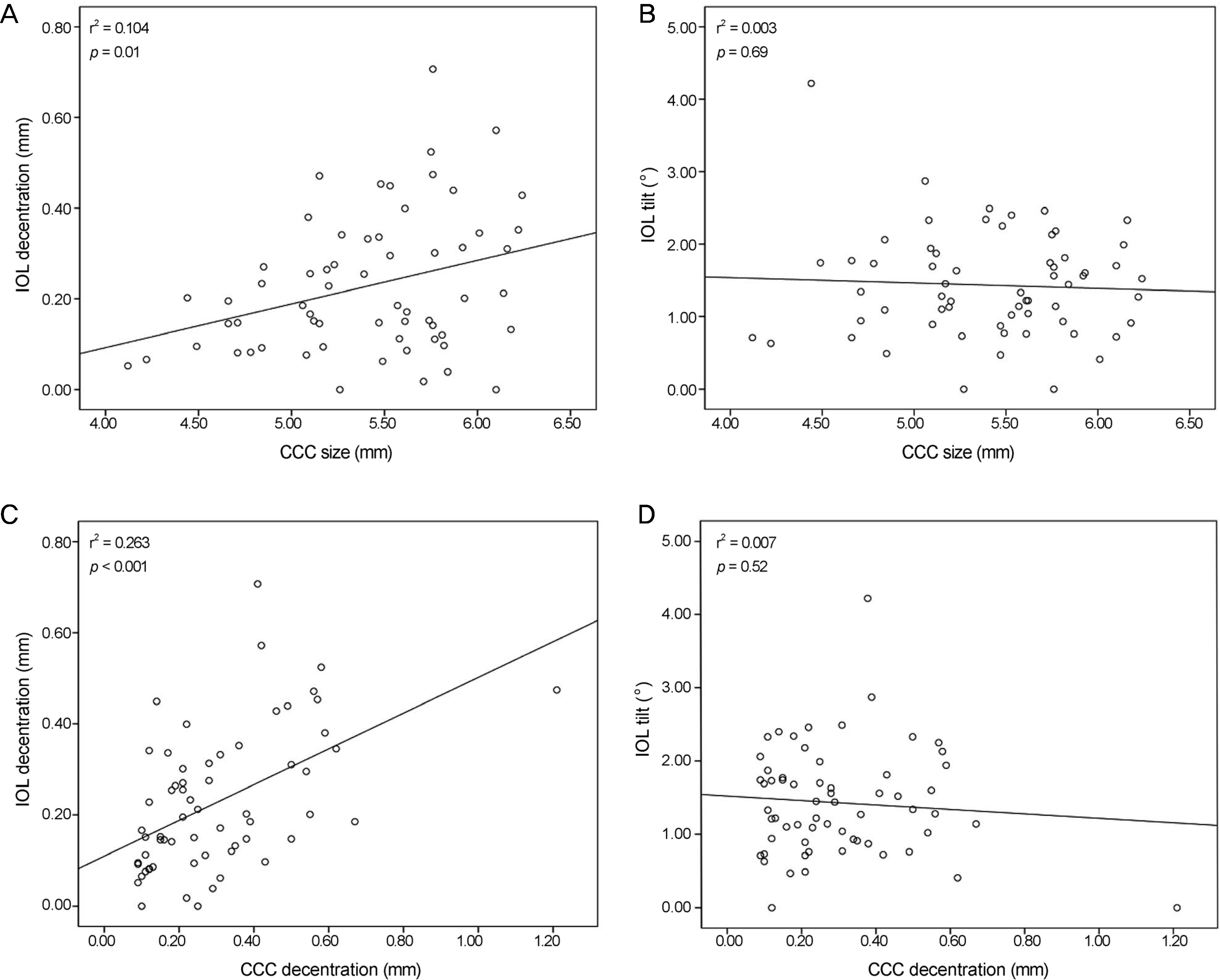 | Figure 4.Plots of correlation of CCC with IOL. CCC size and IOL decentration (A), CCC size and IOL tilt (B), CCC decentration and IOL decentration (C), CCC decentration and IOL tilt (D). r² = coefficient of determination; CCC = continuous curvilinear capsulorhexis; IOL = intraocular lens. |
Table 1.
Patient characteristics
Table 2.
Parameters of continuous curvilinear capsulorhexis and intraocular lens
| Parameters | Mean ± SD | Min, Max |
|---|---|---|
| CCC size (mm) | 5.40 ± 0.51 | 4.12, 6.24 |
| CCC decentration (mm) | 0.30 ± 0.19 | 0.09, 1.21 |
| IOL decentration (mm) | 0.23 ± 0.15 | 0.00, 0.71 |
| IOL tilt (°) | 1.43 ± 0.73 | 0.00, 4.22 |
Table 3.
Postoperative clinical outcomes and p-value of linear regression between intraocular lens position and postoperative clinical outcomes
| Mean ± SD | Min, Max | p-value* | p-value† | |
|---|---|---|---|---|
| Postoperative UCVA (decimal) | 0.77 ± 0.26 | 0.10, 1.00 | 0.46 | 0.14 |
| Postoperative BCVA (decimal) | 0.95 ± 0.12 | 0.50, 1.00 | 0.65 | 0.85 |
| Postoperative MRSE (D, emme target) | −0.17 ± 0.51 | −1.75, 0.50 | 0.21 | 0.27 |
| Postoperative MRSE (D, near target) | −2.56 ± 1.10 | −4.00, −0.25 | 0.17 | 0.21 |
| Postoperative cylinder (D) | −0.61 ± 0.47 | −2.00, 0.00 | 0.34 | 0.07 |
| Coma (micron) | 0.46 ± 0.38 | 0.08, 2.07 | 0.35 | 0.87 |
| Trefoil (micron) | 0.51 ± 0.33 | 0.07, 2.01 | 0.15 | 0.72 |
| HoA (micron) | 0.30 ± 0.16 | 0.08, 0.92 | 0.43 | 0.58 |
| SA (micron) | 0.25 ± 0.19 | 0.02, 1.40 | 0.71 | 0.88 |
SD = standard deviation; Min = minimum; Max = maximum; UCVA = uncorrected visual acuity; BCVA = best-corrected visual acuity; MRSE = manifest refraction spherical equivalent; D = diopter; HoA = high-order aberration; SA = spherical aberration.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download