Abstract
Purpose
To evaluate the effect of orbital wall reconstruction with absorbable osteoconductive unsintered hydroxyapatite/poly L-lactide by assessment of the orbital volume via orbital computed tomography.
Methods
24 patients who followed up at least 6 months after orbital wall reconstruction with unsintered hydroxyapatite/poly L-lactide were included. Retrospective clinical chart reviews for clinical manifestations and complications were performed, and orbital volume measurements were taken using the Eclipse Treatment Planning System (ver.13.0, Varian Medical System Inc., Palo Alto, CA, USA) through orbital computed tomography, which were taken before operation, right after operation, and at last follow up.
Results
Fourteen patients (58.3%) showed diplopia and extraocular muscle movement limitation preoperatively. Diplopia was resolved at last follow up and extraocular muscle movement limitation was improved at postoperative 6 months for all cases. The mean volumes of the fractured orbit and the unaffected orbit before operation were 23.62 ± 0.45 cm3 and 21.95 ± 1.01 cm3, respectively (p = 0.003). The mean volumes of the fractured orbit and the unaffected orbit right after operation were 21.65 ± 0.91 cm3 and 21.78 ± 0.83 cm3, respectively (p = 0.542). The mean volumes of the fractured orbit and the unaffected orbit at last follow up were 21.84 ± 0.93 cm3 and 21.81 ± 0.91 cm3, respectively (p = 0.889).
Go to : 
References
1. Jeon SY, Kim C, Ma Y, Hwang E. Microsurgical intranasal reconstruction of isolated blowout fractures of the medial orbital wall. Laryngoscope. 1996; 106:910–3.

2. Choi JC, Fleming JC, Aitken PA, Shore JW. Porous polyethylene channel implants: a modified porous polyethylene sheet implant designed for repairs of large and complex orbital wall fractures. Ophthal Plast Reconstr Surg. 1999; 15:56–66.
3. Block MS, Kent JN. Correction of vertical orbital dystopia with a hydroxyapatite orbital floor graft. J Oral Maxillofac Surg. 1988; 46:420–5.
4. Jordan DR, St Onge P, Anderson RL, et al. Complications associated with alloplastic implants used in orbital fracture repair. Ophthalmology. 1992; 99:1600–8.

5. Scales JT. Discussion on metals and synthetic materials in relation to soft tissues; Tissue reaction to synthetic materials. Proc R Soc Med. 1953; 46:647.
6. Shikinami Y, Matsusue Y, Nakamura T. The complete process of bioresorption and bone replacement using devices made of forged composites of raw hydroxyapatite particles/poly l-lactide (F-u-HA/PLLA). Biomaterials. 2005; 26:5542–51.
7. Shikinami Y, Okuno M. Bioresorbable devices made of forged composites of hydroxyapatite (HA) particles and poly-L-lactide (PLLA): Part I. Basic characteristics. Biomaterials. 1999; 20:859–77.
8. Shikinami Y, Okuno M. Bioresorbable devices made of forged composites of hydroxyapatite (HA) particles and poly L-lactide (PLLA). Part II: practical properties of miniscrews and miniplates. Biomaterials. 2001; 22:3197–211.

9. Oum BS. Eye Exam. 3rd ed.Goyang: Naewaehaksool;2013. p. 146–7.
10. Mesbahi A, Thwaites DI, Reilly AJ. Experimental and Monte Carlo evaluation of eclipse treatment planning system for lung dose calculations. Rep Pract Oncol Radiother. 2006; 11:123–33.

11. Ye J, Kook KH, Lee SY. Evaluation of computer-based volume measurement and porous polyethylene channel implants in reconstruction of large orbital wall fractures. Invest Ophthalmol Vis Sci. 2006; 47:509–13.

12. Matsuda N, Kaji F. Form control of crystals and aggregation of hydroxyapatites. Kokubo T, Nakamura T, Miyaji F, editors. Bioceramics. Oxford: Pergamon Press;1996. 9:chap. 9.
13. Ducheyne P, Qiu Q. Bioactive ceramic: the effect of surface reactivity on bone formation and bone cell function. Biomaterials. 1999; 20:2287–303.
14. Matsusue Y, Niibayashi H, Aoki Y. Bone fusion using HA/PLLA composite, a material characterized by its excellent resorbability in the living body. J Orthop Surg (Hong Kong). 1999; 50:1405–11.
15. Hayashi M, Muramatsu H, Sato M, et al. Surgical treatment of facial fracture by using unsintered hydroxyapatite particles/poly l-lactide composite device (OSTEOTRANS MX(R)): a clinical study on 17 cases. J Craniomaxillofac Surg. 2013; 41:783–8.
Go to : 
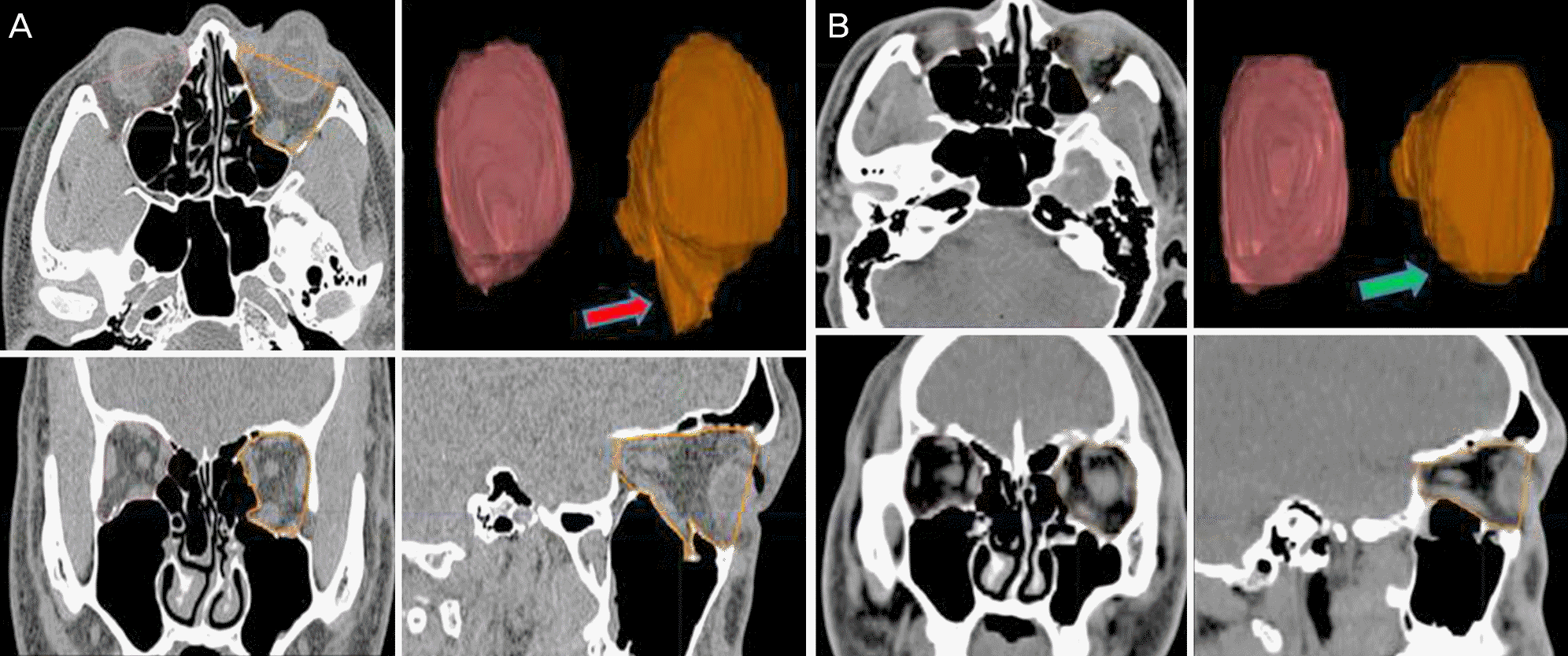 | Figure 1.Example of an orbital volume measurement using the Eclipse Treatment Planning System. (A) Preoperative, (B) postoperative. Axial plane: the anterior orbital boundary was defined by a straight line connecting the medial and the lateral orbital rims, with the posterior limit being the orbital apex. Coronal plane: the anterior orbital boundary was defined as the CT slice in which 50% of the inferior orbital rim was visible, with the posterior limit being the orbital apex. Sagittal plane: the anterior orbital boundary was defined by a straight line connecting the superior and inferior orbital rims, with the posterior limit being the orbital apex. The areas of these outlines were measured on each scan and summed for orbital volume. A red arrow indicates the left inferior wall fracture and herniated orbital tissue before operation. A green arrow indicates the reconstructed left inferior wall fracture. The figure shows a reduction in the displaced orbital wall and herniated orbital tissue compared with the preoperative status. CT = computed tomography. |
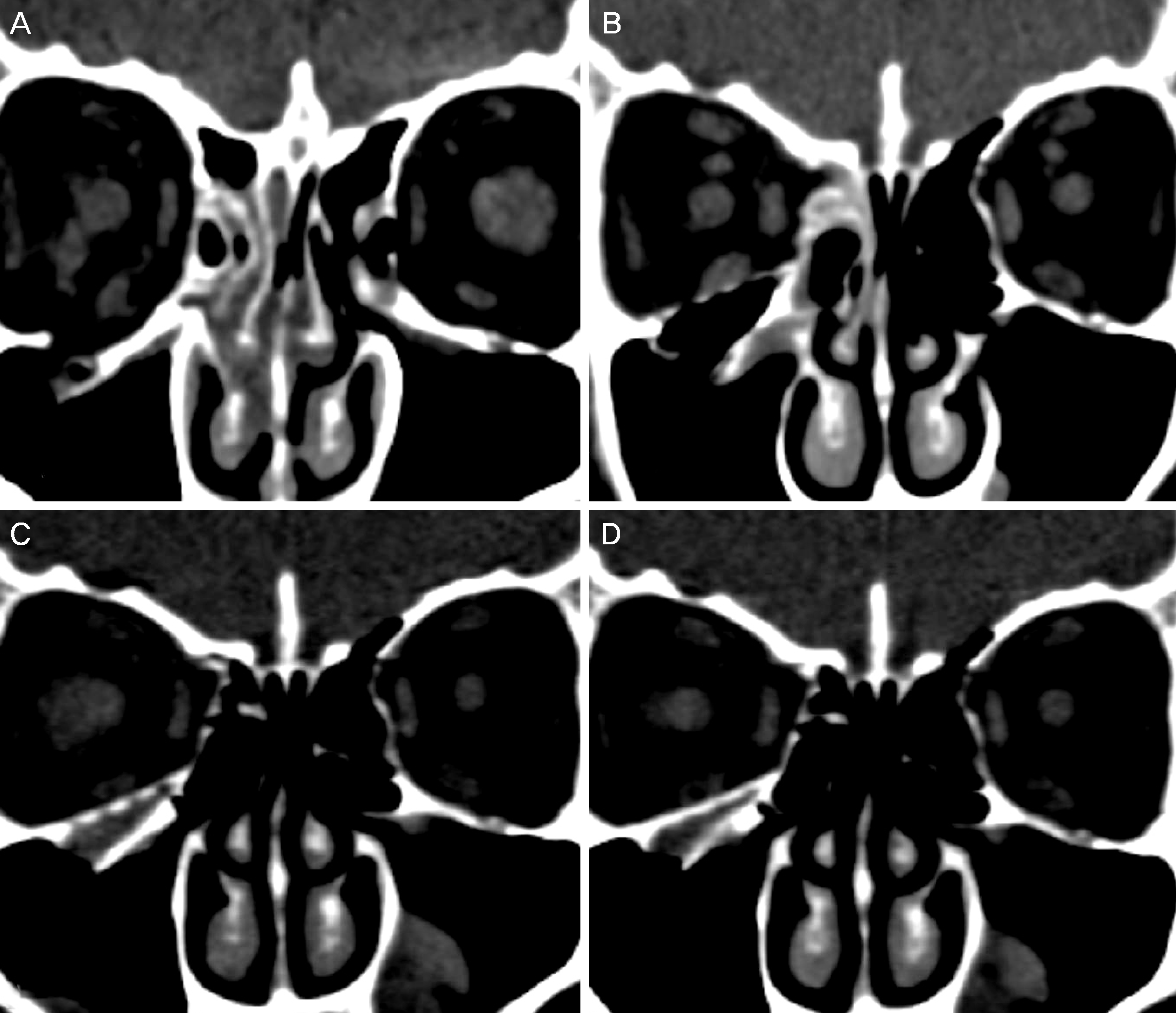 | Figure 2.Serial orbital computed tomography. (A) Preoperative orbital CT shows a right floor fracture with herniated tissue into the maxillary sinus. Postoperative serial orbital CTs show a radiopaque orbital implant right after operation (B), postoperative 6 months (C), and postoperative 12 months (D). (D) Postoperative 12 month CT shows the implant is thickened and pores are filled with adjacent bone density material. This patient's orbital wall reconstruction rate was 101.4% right after operation (B) and 100.5% at postoperative 12 months (D). CT = computed tomography. |
Table 1.
Preoperative and postoperative EOM limitation and diplopia of patients
|
Preoperative |
Postoperative |
|||||
|---|---|---|---|---|---|---|
|
6 months |
Final follow up |
|||||
| EOM limitation | Diplopia* | EOM limitation | Diplopia | EOM limitation | Diplopia | |
| 1 | −2 | Gr III | 0 | Gr I | 0 | Absence |
| 2 | −1 | Gr II | 0 | Absence | 0 | Absence |
| 3 | −1 | Gr II | 0 | Absence | 0 | Absence |
| 4 | −1 | Gr II | 0 | Absence | 0 | Absence |
| 5 | −1 | Gr II | 0 | Absence | 0 | Absence |
| 6 | −2 | Gr III | 0 | Absence | 0 | Absence |
| 7 | −2 | Gr III | 0 | Gr I | 0 | Absence |
| 8 | −1 | Gr II | 0 | Absence | 0 | Absence |
| 9 | −2 | Gr III | 0 | Gr I | 0 | Absence |
| 10 | −2 | Gr III | 0 | Absence | 0 | Absence |
| 11 | −1 | Gr I | 0 | Absence | 0 | Absence |
| 12 | −1 | Gr I | 0 | Absence | 0 | Absence |
| 13 | −2 | Gr III | 0 | Gr I | 0 | Absence |
| 14 | −3 | Gr III | 0 | Gr I | 0 | Absence |
“0” limitation: no limitation with light reflection position beyond limbus. “-1” limitation: limitation with corneal reflection on limbus. “-2” limitation: limitation with corneal reflection between pupil margin and limbus. “-3” limitation: limitation with corneal reflection in contrary pupil margin. “-4” limitation: limitation with inability of the eye to move.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download