Abstract
Purpose
To evaluate the effects of short-term prostaglandin analogues treatment on the corneal biomechanics of patients with normal tension glaucoma.
Methods
This study included 52 eyes of 52 patients who were diagnosed with normal tension glaucoma. All patients were divided into two groups; one group (27 eyes) received tafluprost while the other group (25 eyes) received travoprost. Intraocular pressure, Biomechanical properties were measured by using goldmann applanation tonometer, ocular response analyzer before treatment and at 8-week after treatment.
Results
The mean decrease in intraocular pressure, Goldmann-correlated IOP (IOPg), corneal-compensated intraocular pressure by using Goldmann applanation tonometer, and Ocular response analyzer were statistically significant in total patients, tafluprost, and travoprost group after using prostaglandin analogues (p < 0.001, p < 0.001, p < 0.001, respectively). Corneal hysteresis showed no statistical differences after treatment in total, tafluprost and travoprost group but corneal resistance factor (CRF) showed statistically significant decrease after using prostaglandin analogues in total, tafluprost, and travoprost group (p < 0.001, p = 0.025, p < 0.001). Upon multivariate analysis, the higher initial IOPg and the lower initial CRF checked, the variation of CRF (CRF in baseline – CRF at 8 weeks) got higher (β = 0.134, p = 0.017).
Conclusions
It is needed to carefully monitor and evaluate the effects of prostaglandin analogues on intraocular pressure associated with initial intraocular pressure and the changes of CRF after prostaglandin treatment in normal tension glaucoma patients. CRF is sensitive factor to short-term changes of intraocular pressure after prostaglandin analogues treatment, and it is required to consider the properties of CRF when we evaluate between progression of glaucoma and corneal biomechanical properties.
References
1. Hoyng PF, Kitazawa Y. Medical treatment of normal tension glaucoma. Surv Ophthalmol. 2002; 47(Suppl 1):S116–24.

2. Kim CS, Seong GJ, Lee NH, et al. Prevalence of primary open-angle glaucoma in central South Korea the Namil study. Ophthalmology. 2011; 118:1024–30.
3. Rhew JY, Choi KR. Corneal biomechanical properties of normal tension glaucoma in young patients evaluated with the ocular response analyzer. J Korean Ophthalmol Soc. 2013; 54:280–8.

4. Liu Y, Yanai R, Lu Y, et al. Effects of antiglaucoma drugs on collagen gel contraction mediated by human corneal fibroblasts. J Glaucoma. 2006; 15:255–9.

5. Toris CB, Camras CB, Yablonski ME. Effects of PhXA41, a new prostaglandin F2 alpha analog, on aqueous humor dynamics in human eyes. Ophthalmology. 1993; 100:1297–304.
6. Feldman RM. Conjunctival hyperemia and the use of topical prostaglandins in glaucoma and ocular hypertension. J Ocul Pharmacol Ther. 2003; 19:23–35.

7. Wu KY, Wang HZ, Hong SJ. Effect of latanoprost on cultured porcine corneal stromal cells. Curr Eye Res. 2005; 30:871–9.

8. Gunvant P, O'Leary DJ, Baskaran M, et al. Evaluation of tonometric correction factors. J Glaucoma. 2005; 14:337–43.

9. Schroeder B, Hager A, Kutschan A, Wiegand W. Measurement of viscoelastic corneal parameters (corneal hysteresis) in patients with primary open angle glaucoma. Ophthalmologe. 2008; 105:916–20.
10. Shah S, Laiquzzaman M, Mantry S, Cunliffe I. Ocular response analyser to assess hysteresis and corneal resistance factor in low tension, open angle glaucoma and ocular hypertension. Clin Experiment Ophthalmol. 2008; 36:508–13.

11. Sullivan-Mee M, Billingsley SC, Patel AD, et al. Ocular Response Analyzer in subjects with and without glaucoma. Optom Vis Sci. 2008; 85:463–70.

12. Wells AP, Garway-Heath DF, Poostchi A, et al. Corneal hysteresis but not corneal thickness correlates with optic nerve surface compliance in glaucoma patients. Inves Ophthalmol Vis Sci. 2008; 49:3262–8.

13. Schachtschabel U, Lindsey JD, Weinreb RN. The mechanism of action of prostaglandins on uveoscleral outflow. Curr Opin Ophthalmol. 2000; 11:112–5.

14. Lindsey JD, Kashiwagi K, Kashiwagi F, Weinreb RN. Prostaglandin action on ciliary smooth muscle extracellular matrix metabolism: implications for uveoscleral outflow. Surv Ophthalmol. 1997; 41(Suppl 2):S53–9.

15. Mietz H, Esser JM, Welsandt G, et al. Latanoprost stimulates secretion of matrix metalloproteinases in tenon fibroblasts both in vitro and in vivo. Invest Ophthalmol Vis Sci. 2003; 44:5182–8.

16. Ocklind A. Effect of latanoprost on the extracellular matrix of the ciliary muscle. A study on cultured cells and tissue sections. Exp Eye Res. 1998; 67:179–91.

17. Oh DJ, Martin JL, Williams AJ, et al. Effect of latanoprost on the expression of matrix metalloproteinases and their tissue inhibitors in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2006; 47:3887–95.

18. Sagara T, Gaton DD, Lindsey JD, et al. Topical prostaglandin F2alpha treatment reduces collagen types I, III, and IV in the monkey uveoscleral outflow pathway. Arch Ophthalmol. 1999; 117:794–801.
19. Wang N, Lindsey JD, Angert M, Weinreb RN. Latanoprost and matrix metal loproteinase-1 in human choroid organ cultures. Curr Eye Res. 2001; 22:198–207.
20. Rasmussen CA, Kaufman PL. Medical therapy for glaucoma: the next 20 years. Journal of Current Glaucoma Practice. 2008; 2:1–5.

21. Medeiros FA, Weinreb RN. Evaluation of the influence of corneal biomechanical properties on intraocular pressure measurements using the ocular response analyzer. J Glaucoma. 2006; 15:364–70.

22. Luce D, Taylor D. Reichert Ocular Response Analyzer Measures Corneal Biomechanical Properties and IOP. 1st ed. New York: Reichert Ophthalmic Instruments Publication;2006. p. 4–7.
23. Agarwal DR, Ehrlich JR, Shimmyo M, Radcliffe NM. The relationship between corneal hysteresis and the magnitude of intraocular pressure reduction with topical prostaglandin therapy. Br J Ophthalmol. 2012; 96:254–7.

24. Congdon NG, Broman AT, Bandeen-Roche K, et al. Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am J Ophthalmol. 2006; 141:868–75.

25. Tsikripis P, Papaconstantinou D, Koutsandrea C, et al. The effect of prostaglandin analogs on the biomechanical properties and central thickness of the cornea of patients with open-angle glaucoma: a 3-year study on 108 eyes. Drug Des Devel Ther. 2013; 7:1149–56.
26. Emre S, Kayikçioğlu O, Ateş H, et al. Corneal hysteresis, corneal resistance factor, and intraocular pressure measurement in patients with scleroderma using the reichert ocular response analyzer. Cornea. 2010; 29:628–31.

27. Ang GS, Bochmann F, Townend J, Azuara-Blanco A. Corneal biomechanical properties in primary open angle glaucoma and normal tension glaucoma. J Glaucoma. 2008; 17:259–62.

28. Noecker RS, Dirks MS, Choplin NT, et al. A six-month randomized clinical trial comparing the intraocular pressure-lowering efficacy of bimatoprost and latanoprost in patients with ocular hypertension or glaucoma. Am J Ophthalmol. 2003; 135:55–63.

29. Park KH, Park SJ, Lee YJ, et al. Ability of peripapillary atrophy parameters to differentiate normal-tension glaucoma from glaucomalike disk. J Glaucoma. 2001; 10:95–101.

30. Woo SJ, Park KH, Kim DM. Comparison of localised nerve fibre layer defects in normal tension glaucoma and primary open angle glaucoma. Br J Ophthalmol. 2003; 87:695–8.

31. Grise-Dulac A, Saad A, Abitbol O, et al. Assessment of corneal biomechanical properties in normal tension glaucoma and comparison with open-angle glaucoma, ocular hypertension, and normal eyes. J Glaucoma. 2012; 21:486–9.

32. Sellem E, Rouland JF, Baudouin C, et al. Predictors of additional intraocular pressure reduction in patients changed to latanoprost/timolol fixed combination. BMC Ophthalmol. 2010; 10:10.

33. De Moraes CV, Hill V, Tello C, et al. Lower corneal hysteresis is associated with more rapid glaucomatous visual field progression. J Glaucoma. 2012; 21:209–13.

34. Brandt JD, Beiser JA, Gordon MO, et al. Central corneal thickness and measured IOP response to topical ocular hypotensive medication in the Ocular Hypertension Treatment Study. Am J Ophthalmol. 2004; 138:717–22.

35. Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120:714–20.
36. Hager A, Loge K, Schroeder B, et al. Effect of central corneal thickness and corneal hysteresis on tonometry as measured by dynamic contour tonometry, ocular response analyzer, and Goldmann tonometry in glaucomatous eyes. J Glaucoma. 2008; 17:361–5.
Figure 1.
Comparison of intraocular pressure (IOP) changes after dosing of tafluprost and travoprost. (A) Intraocular pressure
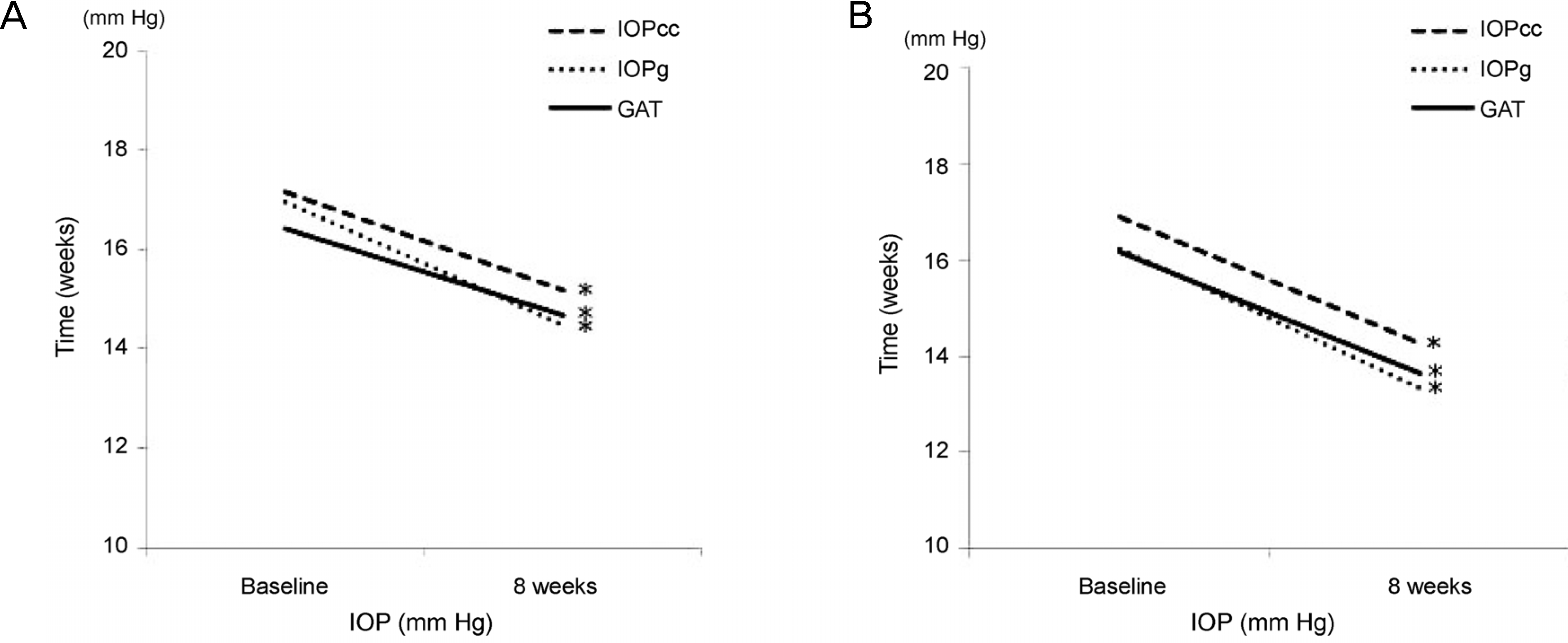
Figure 2.
A relationship between CRF changes and baseline IOPg. A scatter plot illustrates significantly positive relationships between IOPg measurements in baseline and CRF variation (CRF in baseline - CRF in 8 weeks using treatment) for each patient treated with tafluprost or travoprost. CRF = corneal resistance factor; IOPg = Goldmann-correlated intraocular pressure.
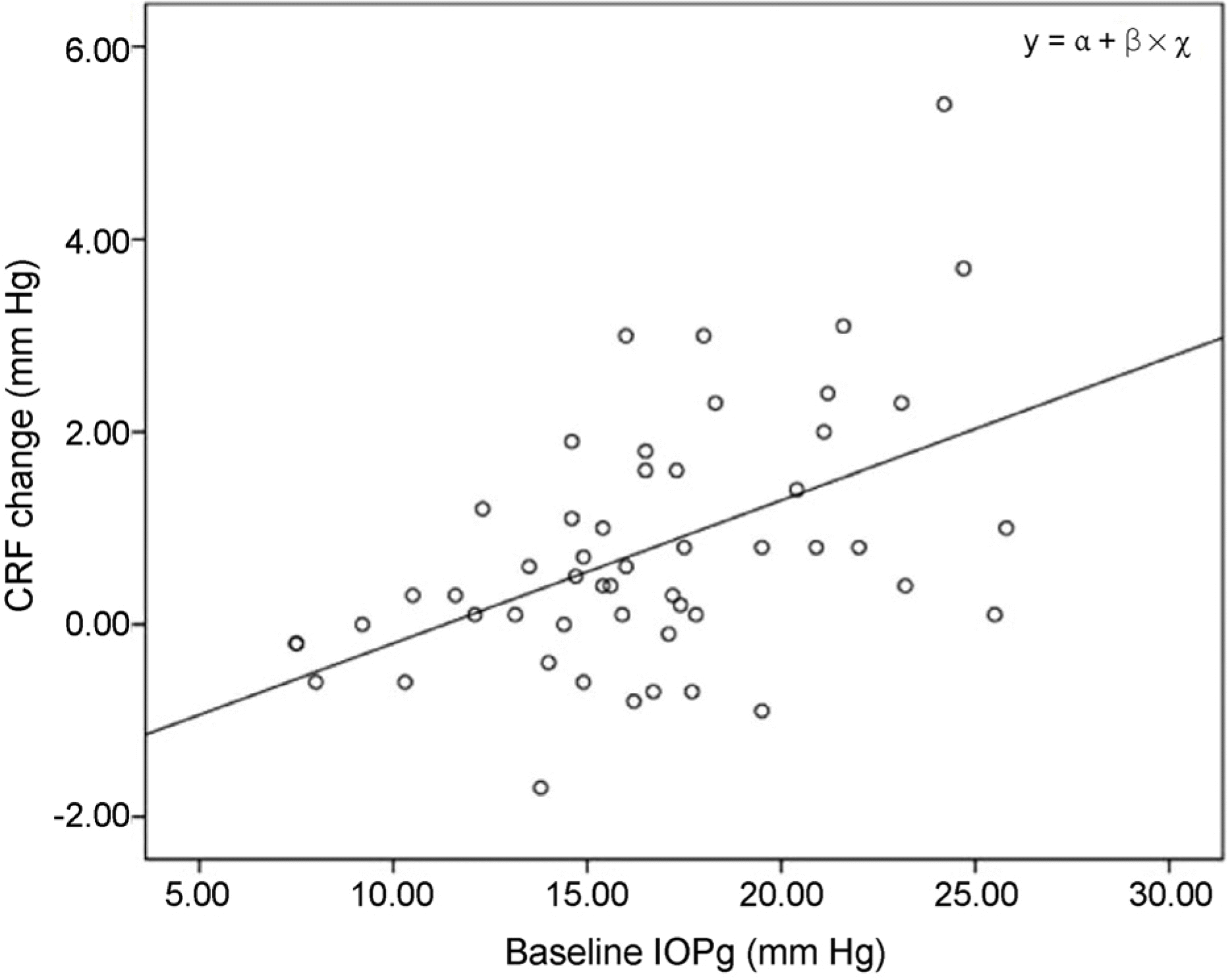
Table 1.
Clinical and ocular characteristics of the normal tension glaucoma patients groups
| Total (n = 52) | Tafluprost (n = 27) | Travoprost (n = 25) | p-value | |
|---|---|---|---|---|
| Age (years) | 53.8 ± 15.2 | 52.3 ± 14 | 55.1 ± 16.3 | 0.301* |
| Female (%) | 44.2 | 43.0 | 41.4 | 0.788† |
| CCT (μm) | 555 ± 39.2 | 555 ± 40 | 554 ± 39.2 | 0.697* |
| Axial length (mm) | 24.84 ± 1.71 | 24.79 ± 1.71 | 24.89 ± 1.75 | 0.800* |
| Baseline IOP (mm Hg) | 16.29 ± 3.70 | 16.44 ± 3.68 | 16.15 ± 3.79 | 0.795* |
| SE (diopters) | −2.43 ± 3.22 | −2.76 ± 3.36 | −2.12 ± 3.12 | 0.442* |
| Visual field index | 82.58 ± 15.38 | 81.39 ± 14.99 | 83.69 ± 15.95 | 0.258* |
| MD (dB) | −7.39 ± 4.96 | −7.4 ± 5.04 | −7.38 ± 4.98 | 0.491* |
| PSD (dB) | 7.34 ± 4.62 | 7.71 ± 4.36 | 6.99 ± 4.91 | 0.232* |
Table 2.
Corneal biomechanical parameters by Ocular Response Analyzer in normal tension glaucoma patient groups; baseline and after 8 weeks of total patients, tafloprust, and travoprost
| Baseline | 8 weeks | p-value* | |
|---|---|---|---|
| Total | |||
| IOPcc (mm Hg) | 17.02 ± 4.13 | 14.70 ± 3.26 | <0.001 |
| IOPg (mm Hg) | 16.59 ± 4.48 | 13.90 ± 3.57 | <0.001 |
| GAT (mm Hg) | 16.29 ± 3.70 | 14.13 ± 3.14 | <0.001 |
| CH (mm Hg) | 10.29 ± 1.65 | 10.29 ± 1.79 | 0.981 |
| CRF (mm Hg) | 10.64 ± 2.07 | 9.85 ± 2.08 | <0.001 |
| Tafluprost | |||
| IOPcc (mm Hg) | 17.16 ± 4.10 | 15.20 ± 3.41 | 0.001 |
| IOPg (mm Hg) | 16.97 ± 4.14 | 14.50 ± 3.57 | <0.001 |
| GAT (mm Hg) | 16.44 ± 3.68 | 14.68 ± 3.4 | 0.002 |
| CH (mm Hg) | 10.49 ± 1.65 | 10.36 ± 1.81 | 0.542 |
| CRF (mm Hg) | 10.92 ± 1.80 | 10.07 ± 1.98 | 0.025 |
| Travoprost | |||
| IOPcc (mm Hg) | 16.87 ± 4.23 | 14.25 ± 3.10 | 0.001 |
| IOPg (mm Hg) | 16.24 ± 4.84 | 13.34 ± 3.53 | <0.001 |
| GAT (mm Hg) | 16.15 ± 3.79 | 13.63 ± 2.86 | 0.033 |
| CH (mm Hg) | 10.11 ± 1.67 | 10.23 ± 1.80 | 0.633 |
| CRF (mm Hg) | 10.39 ± 2.30 | 9.66 ± 2.19 | <0.001 |
Table 3.
Result of multiple linear regression analysis with CRF variations obtained with Ocular Response Analyzer as dependent variable in normal tension glaucoma patients being treated with tafluprost or travoprost
|
CRF variations (initial CRF–final CRF) |
||
|---|---|---|
| β | p-value | |
| Model 1 | ||
| Age | −0.019 | 0.192 |
| IOPcc | 0.101 | 0.118 |
| GAT | 0.006 | 0.936 |
| AXL | 0.076 | 0.557 |
| Model 2 | ||
| Age | −0.018 | 0.211 |
| IOPg | 0.134 | 0.017* |
| GAT | −0.034 | 0.610 |
| AXL | 0.104 | 0.380 |
Multiple regression models for analyzing relationship between CRF variations and other clinical variables including intraocular pressure parameters (IOPcc in Model 1, IOPg in Model 2).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download