Abstract
Purpose
To evaluate the surgical results of Ahmed valve implant surgery with adjunctive mitomycin C and selective postoperative 5-fluorouracil (5-FU) subconjunctival injection with a needling procedure.
Methods
In this retrospective study, 40 eyes of 40 patients who had undergone Ahmed valve implant surgery with adjunctive mitomycin C were observed for at least 1 year. The Ahmed valve was implanted after 5-minute application of 0.04% mitomycin C. Selective 5-FU injection with a needling procedure was performed during the follow- up period based on intraocular pressure (IOP). Hypertensive phase was defined as IOP higher than 21 mm Hg within 3 months after operation. IOP higher than 18 mm Hg regardless of IOP-lowering medications at 2 consecutive visits was considered to be a surgical failure.
Results
The mean follow-up period was 35.5 ± 12.4 months. Preoperative intraocular pressure was 32.8 ± 7.5 mm Hg, which decreased to 14.0 ± 4.2 mm Hg postoperatively. The number of glaucoma medications decreased significantly from 3.8 ± 0.5 to 2.0 ± 1.0. Eleven of 40 eyes (27.5%) experienced hypertensive phase at 6.0 ± 3.1 weeks after surgery. Kaplan-Meier survival analysis showed cumulative probability of surgical success rates of 82.5%, 79.6%, 72.7%, and 58.8% at 1, 2, 3, and 4 postoperative years respectively. There were no risk factors that affecting surgical failure except age (hazard ratio = 0.17, p = 0.02). Conclusions: Ahmed valve implant surgery with adjunctive mitomycin C and selective 5-FU injection with a needling procedure showed good success in refractory glaucoma.
Go to : 
References
1. Taglia DP, Perkins TW, Gangnon R, et al. Comparison of the Ahmed glaucoma valve, the Krupin eye valve with disk, and the double-plate Molteno implant. J Glaucoma. 2002; 11:347–53.

2. Lim KS, Allan BD, Lloyd AW, et al. Glaucoma drainage devices; past, present, and future. Br J Ophthalmol. 1998; 82:1083–9.

3. Wu SC, Huang SC, Lin KK. Clinical experience with the Ahmed glaucoma valve implant in complicated glaucoma. Chang Gung Med J. 2003; 26:904–10.
4. Coleman AL, Wilson MR, Tam M, et al. Initial clinical experience with the Ahmed glaucoma valve implant–correction. Am J Ophthalmol. 1995; 120:684.

5. Bae JS, Lee NH, Kim HK, Sohn YH. Comparison of safety and efficacy between silicone and polypropylene Ahmed glaucoma valves. J Korean Ophthalmol Soc. 2008; 49:791–7.

6. Kurnaz E, Kubaloglu A, Yilmaz Y, et al. The effect of adjunctive Mitomycin C in Ahmed glaucoma valve implantation. Eur J Ophthalmol. 2005; 15:27–31.

7. Alvarado JA, Hollander DA, Juster RP, Lee LC. Ahmed valve implantation with adjunctive mitomycin C and 5-fluorouracil: long-term outcomes. Am J Ophthalmol. 2008; 146:276–84.

8. Susanna R Jr, Nicolela MT, Takahashi WY. Mitomycin C as adjunctive therapy with glaucoma implant surgery. Ophthalmic Surg. 1994; 25:458–62.

9. Kook MS, Yoon J, Kim J, Lee MS. Clinical results of Ahmed glaucoma valve implantation in refractory glaucoma with adjunctive mitomycin C. Ophthalmic Surg Lasers. 2000; 31:100–6.

10. Sidoti PA, Dunphy TR, Baerveldt G, et al. Experience with the Baerveldt glaucoma implant in treating neovascular glaucoma. Ophthalmology. 1995; 102:1107–18.

11. Kee C. Prevention of early postoperative hypotony by partial ligation of silicone tube in Ahmed glaucoma valve implantation. J Glaucoma. 2001; 10:466–9.

12. Lee JJ, Park KH, Kim DM, Kim TW. Clinical outcomes of Ahmed glaucoma valve implantation using tube ligation and removable external stents. Korean J Ophthalmol. 2009; 23:86–92.

13. Netland PA, Ishida K, Boyle JW. The Ahmed Glaucoma Valve in patients with and without neovascular glaucoma. J Glaucoma. 2010; 19:581–6.

14. Souza C, Tran DH, Loman J, et al. Long-term outcomes of Ahmed glaucoma valve implantation in refractory glaucomas. Am J Ophthalmol. 2007; 144:893–900.

15. Kim JJ, Shin JP. Long-term results of Ahmed valve implantation in neovascular glaucoma and the effects of intracameral bevacizumab. J Korean Ophthalmol Soc. 2013; 54:757–65.

16. Lee SH, Ma KT, Hong YJ. Outcome of Ahmed valve implantation in refractory glaucoma. J Korean Ophthalmol Soc. 2007; 48:83–90.
17. Lim SH, Seo WM, Park JJ, Yun SU. Ahmed valve implantation with adjunctive mitomycin C and 5-fluorouracil: outcomes at 2 years. J Korean Ophthalmol Soc. 2011; 52:1470–7.

18. Yoon HJ, Park JJ. Ahmed valve implantation with adjunctive mitomycin C and 5-fluorouracil: outcomes at one year. J Korean Ophthalmol Soc. 2010; 51:227–33.

19. Shaarawy TM, Sherwood MB, Grehn F. Guidelines on Design and Reporting of Glaucoma Surgical Trials. Amsterdam: Kugler Publications. 2009; 17.
20. Lee EK, Yun YJ, Lee JE, et al. Changes in corneal endothelial cells after Ahmed glaucoma valve implantation: 2-year follow-up. Am J ophthalmol. 2009; 148:361–7.

21. McDermott ML, Swendris RP, Shin DH, et al. Corneal endothelial cell counts after Molteno implantation. Am J Ophthalmol. 1993; 115:93–6.

22. Ayyala RS, Zurakowski D, Smith JA, et al. A clinical study of the Ahmed glaucoma valve implant in advanced glaucoma. Ophthalmology. 1998; 105:1968–76.
23. Huang MC, Netland PA, Coleman AL, et al. Intermediate-term clinical experience with the Ahmed Glaucoma Valve implant. Am J Ophthalmol. 1999; 127:27–33.
24. Coleman AL, Smyth RJ, Wilson MR, Tam M. Initial clinical experience with the Ahmed glaucoma valve implant in pediatric patients. Arch Ophthalmol. 1997; 115:186–91.

25. Siegner SW, Netland PA, Urban RC Jr, et al. Clinical experience with the Baerveldt glaucoma drainage implant. Ophthalmology. 1995; 102:1298–307.

26. Won HJ, Sung KR. Hypertensive phase following silicone plate Ahmed glaucoma valve implantation. J Glaucoma. 2015; Mar 13. [Epub ahead of print].

27. Nouri-Mahdavi K, Caprioli J. Evaluation of the hypertensive phase after insertion of the Ahmed glaucoma valve. Am J Ophthalmol. 2003; 136:1001–8.

28. Krishna R, Godfrey DG, Budenz DL, et al. Intermediate-term outcomes of 350-mm(2) Baerveldt glaucoma implants. Ophthalmology. 2001; 108:621–6.
29. Minckler DS, Heuer DK, Hasty B, et al. Clinical experience with the single-plate Molteno implant in complicated glaucomas. Ophthalmology. 1988; 95:1181–8.

30. Eibschitz-Tsimhoni M, Schertzer RM, Musch DC, Moroi SE. Incidence and management of encapsulated cysts following Ahmed glaucoma valve insertion. J Glaucoma. 2005; 14:276–9.

31. Chen PP, Palmberg PF. Needling revision of glaucoma drainage device filtering blebs. Ophthalmology. 1997; 104:1004–10.

32. Lama PJ, Fechtner RD. Antifibrotics and wound healing in glaucoma surgery. Surv Ophthalmol. 2003; 48:314–46.

Go to : 
 | Figure 1.Intraocular pressure following Ahmed valve implantation with adjunctive intraoperative mitomycin C and selective postoperative 5-fluorouracil injection and needling revision. |
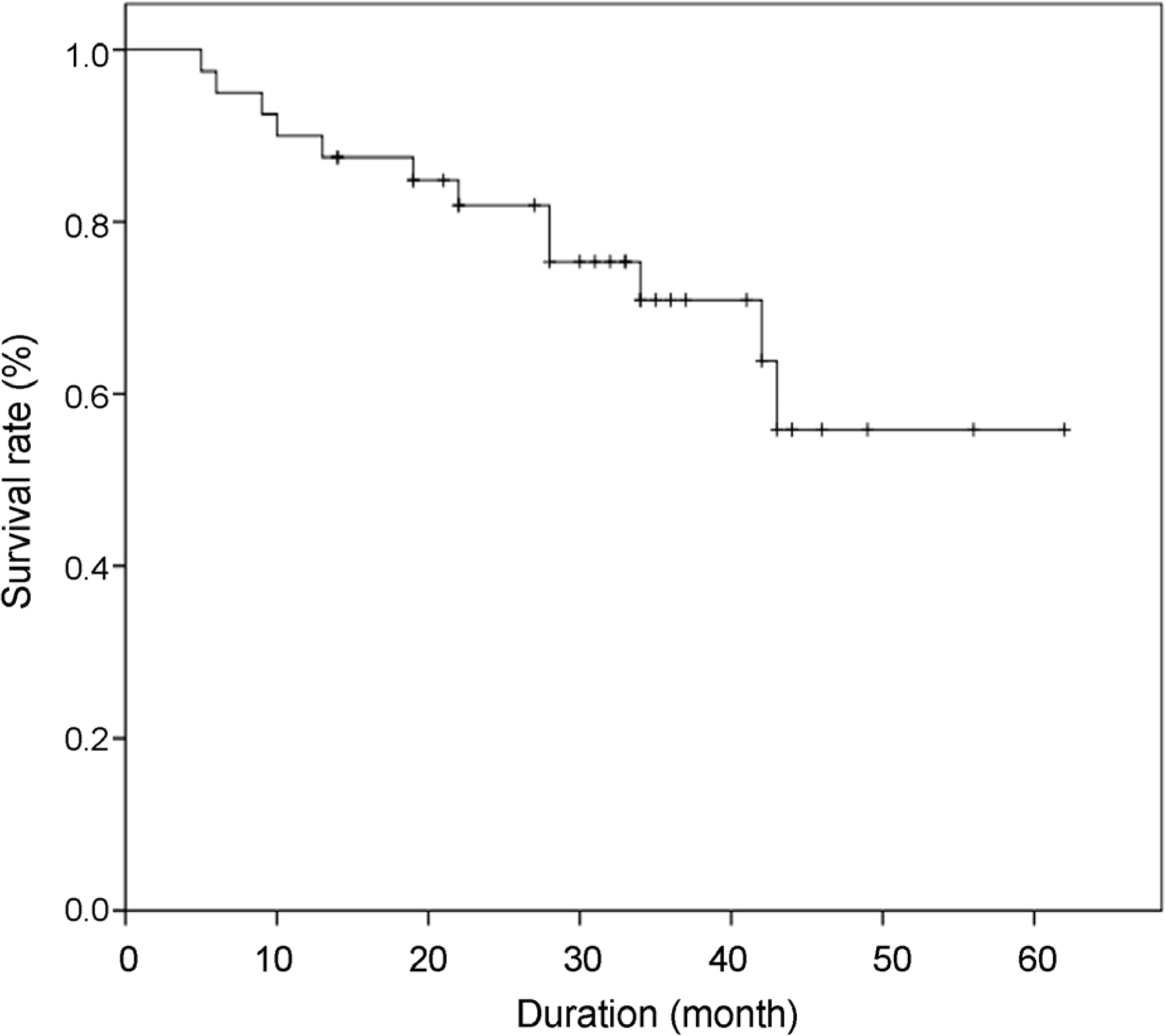 | Figure 2.Kaplan-Meier curve of the cumulative probability of valve success for Ahmed valve-implanted eyes. Study group (40 eyes) received Ahmed valve implantation and intraoperative MMC. Failure was defined as the first occurrence of any of the following events during initial postoperative period: 1) IOP >18 mm Hg or <6 mm Hg for two consecutive visits or <20% IOP reduction from baseline, 2) need for additional surgery to repair a malfunctioning Ahmed valve except 5-fluorouracil injection, or 3) serious postoperative complications including visual acuity loss. MMC = mitomycin C; IOP = intraocular pressure. |
Table 1.
Baseline characteristics for Ahmed valve-implanted eyes
Table 2.
U nivariate Cox regression analyses of predictors of surgical failure with the Ahmed valve implantation
Table 3.
Multivariate Cox regression analyses of predictors of surgical failure with the Ahmed valve implantation*
| Variable | HR | 95% CI | p-value |
|---|---|---|---|
| Age (years) | |||
| 55≤ vs. <55 (ref.) | 0.17 | 0.04-0.81 | 0.02 |
| Gender | |||
| Male vs. Female (ref.) | 0.45 | 0.14-1.45 | 0.18 |
| Preop VA (log MAR) | 0.56 | 0.26-1.17 | 0.12 |
Table 4.
Univariate logistic regression analyses of predictors of visual acuity loss with the AGV implantation
Table 5.
Complications after implantation of Ahmed valves
| Complication | No. of patients (n, %) |
|---|---|
| Hyphema | 4 (10.0) |
| Hypotony | 11 (27.5) |
| Choroidal detachment | 4 (10.0) |
| Shallow A/C | 3 (7.5) |
| Tube reposition | 3 (7.5) |
| Tube exposure | 2 (5.0) |
| Hypertensive phase | 11 (27.5) |
Table 6.
Previously published estimated probability of valve success from studies of Ahmed valve implantation
|
Postoperative year |
Definition of failure (consecutive visits)* | Subject |
Antimetabolite |
Plate | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1st year | 2nd year | 3rd year | 5th year | MMC | 5-FU | ||||
| Netland et al13 | 0.73 | 0.62 | 0.20 | >21 (2) mm Hg | NVG | − | − | Silicone/polypropylene | |
| 0.89 | 0.81 | 0.81 | Refractory glaucoma without NVG | − | − | ||||
| Souza et al14 | 0.8 | 0.73 | 0.63 | 0.49 | >21 (2) mm Hg or <15% | Refractory glaucoma | − | Silicone/polypropylene | |
| Kim and Shin15 | 0.88 | 0.71 | 0.62 | >21 (2) mm Hg | NVG | − | − | ||
| Lee et al16 | 0.58 | 0.47 | 0.4 | >21 mm Hg or <30% | Refractory glaucoma | − | + | Polypropylene | |
| Lim et al17 | 0.38 | 0.19 | >18 (3) mm Hg or <20% | NVG | − | − | Silicone | ||
| 0.83 | 0.44 | + | + | ||||||
| Yoon and Park18 | 0.76 | >18 (3) mm Hg or <20% | NVG | + | + | Silicone | |||
| Alvarado et al7 | 0.92 | 0.87 | 0.82 | 0.72 | >18 (3) mm Hg or <20% | Refractory glaucoma | + | + | Polypropylene |
| This study | 0.85 | 0.79 | 0.72 | 0.58 (4 year) | >18 (2) mm Hg or <20% | Refractory glaucoma | + | + | Silicone |
Table 7.
Rate and onset of postoperative hypertensive phase




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download