Abstract
Purpose
In this study we compared the clinical outcomes of idiopathic epiretinal membrane (ERM) surgery according to the use of indocyanine green (ICG) and ICG exposure time.
Methods
The medical records of 76 patients with an idiopathic ERM that underwent vitrectomy and ERM and internal limiting membrane (ILM) removal were reviewed. We compared the results (best corrected visual acuity [BCVA, log MAR] and central macular thickness [CMT, μm]) of idiopathic ERM surgeries using ILM peeling with (group I, 39 eyes) and without ICG (group II, 37 eyes). Additionally, the correlation of ICG exposure time and clinical outcomes in group I was analyzed.
Results
Gender, age, lens state, preoperative BCVA, and preoperative CMT were not significantly different between the two groups. The postoperative BCVA was significantly improved in both groups but the difference was not statistically significant. The postoperative CMT was significantly improved in both groups and the change amount of group I was more larger than group II. Additionally, ICG exposure time was not significantly correlated with changes of BCVA and CMT.
Go to : 
References
1. Kawasaki R, Wang JJ, Sato H, et al. Prevalence and associations of epiretinal membranes in an adult Japanese population: the Funagata study. Eye (Lond). 2009; 23:1045–51.

2. Klein R, Klein BE, Wang Q, Moss SE. The epidemiology of epiretinal membranes. Trans Am Ophthalmol Soc. 1994; 92:403–25. discussion 425-30.
3. Mitchell P, Smith W, Chey T, et al. Prevalence and associations of epiretinal membranes. The Blue Mountains Eye Study, Australia. Ophthalmology. 1997; 104:1033–40.
4. Poliner LS, Olk RJ, Grand MG, et al. Surgical management of premacular fibroplasia. Arch Ophthalmol. 1988; 106:761–4.

5. de Bustros S, Thompson JT, Michels RG, et al. Vitrectomy for idiopathic epiretinal membranes causing macular pucker. Br J Ophthalmol. 1988; 72:692–5.

6. de Bustros S, Rice TA, Michels RG, et al. Vitrectomy for macular pucker. Use after treatment of retinal tears or retinal detachment. Arch Ophthalmol. 1998; 106:758–60.
7. Pesin SR, Olk RJ, Grand MG, et al. Vitrectomy for premacular fibroplasia. Prognostic factors, long-term follow-up, and time course of visual improvement. Ophthalmology. 1991; 98:1109–14.
8. Kwok AK, Lai TY, Li WW, et al. Indocyanine green-assisted internal limiting membrane removal in epiretinal membrane surgery: a clinical and histologic study. Am J Ophthalmol. 2004; 138:194–9.

9. Machemer R. The surgical removal of epiretinal macular membranes (macular puckers) (author's transl). Klin Monbl Augenheilkd. 1978; 173:36–42.
11. Margherio RR, Cox MS Jr, Trese MT, et al. Removal of epimacular membranes. Ophthalmology. 1985; 92:1075–83.

12. McDonald HR, Verre WP, Aaberg TM. Surgical management of idiopathic epiretinal membranes. Ophthalmology. 1986; 93:978–83.

13. Donati G, Kapetanios AD, Pournaras CJ. Complications of surgery for epiretinal membranes. Graefes Arch Clin Exp Ophthalmol. 1998; 236:739–46.

14. Benhamou N, Massin P, Spolaore R, et al. Surgical management of epiretinal membrane in young patients. Am J Ophthalmol. 2002; 133:358–64.
15. Massin P, Paques M, Masri H, et al. Visual outcome of surgery for epiretinal membranes with macular pseudoholes. Ophthalmology. 1999; 106:580–5.

16. Park DW, Dugel PU, Garda J, et al. Macular pucker removal with and without internal limiting membrane peeling: pilot study. Ophthalmology. 2003; 110:62–4.

17. Kwok AKh, Lai TY, Yuen KS. Epiretinal membrane surgery with or without internal limiting membrane peeling. Clin Experiment Ophthalmol. 2005; 33:379–85.

18. Bovey EH, Uffer S, Achache F. Surgery for epimacular membrane: impact of retinal internal limiting membrane removal on functional outcome. Retina. 2004; 24:728–35.
19. Burk SE, Da Mata AP, Snyder ME, et al. Indocyanine green-assisted peeling of the retinal internal limiting membrane. Ophthalmology. 2000; 107:2010–4.
20. Gandorfer A, Messmer EM, Ulbig MW, Kampik A. Indocyanine green selectively stains the internal limiting membrane. Am J Ophthalmol. 2001; 131:387–8.

21. Da Mata AP, Burk SE, Riemann CD, et al. Indocyanine green-assisted peeling of the retinal internal limiting membrane during vitrectomy surgery for macular hole repair. Ophthalmology. 2001; 108:1187–92.

22. Gandorfer A, Haritoglou C, Gass CA, et al. Indocyanine green-assisted peeling of the internal limiting membrane may cause retinal damage. Am J Ophthalmol. 2001; 132:431–3.

23. Lee JE, Yoon TJ, Oum BS, et al. Toxicity of indocyanine green injected into the subretinal space: subretinal toxicity of indocyanine green. Retina. 2003; 23:675–81.
24. Lai MM, Williams GA. Anatomical and visual outcomes of idiopathic macular hole surgery with internal limiting membrane removal using low-concentration indocyanine green. Retina. 2007; 27:477–82.

25. Kwok AK, Lai TY, Yew DT, Li WW. Internal limiting membrane staining with various concentrations of indocyanine green dye under air in macular surgeries. Am J Ophthalmol. 2003; 136:223–30.

26. Haritoglou C, Gandorfer A, Gass CA, Kampik A. Histology of the vitreoretinal interface after staining of the internal limiting membrane using glucose 5% diluted indocyanine and infracyanine green. Am J Ophthalmol. 2004; 137:345–8.

27. Gandorfer A, Haritoglou C, Gandorfer A, Kampik A. Retinal damage from indocyanine green in experimental macular surgery. Invest Ophthalmol Vis Sci. 2003; 44:316–23.

28. Haritoglou C, Gandorfer A, Schaumberger M, et al. Light-absorbing properties and osmolarity of indocyanine-green depending on concentration and solvent medium. Invest Ophthalmol Vis Sci. 2003; 44:2722–9.

29. Ho JD, Chen HC, Chen SN, Tsai RJ. Reduction of indocyanine green-associated photosensitizing toxicity in retinal pigment epithelium by sodium elimination. Arch Ophthalmol. 2004; 122:871–8.
30. Kim MR, Park JH, Sagong M, Chang WH. Effect of solvent in indocyanine green-assisted internal limiting membrane peeling during idiopathic epiretinal membrane surgery. J Korean Ophthalmol Soc. 2014; 55:847–53.

31. Haritoglou C, Gandorfer A, Gass CA, et al. The effect of indocyanine-green on functional outcome of macular pucker surgery. Am J Ophthalmol. 2003; 135:328–37.

32. Sippy BD, Engelbrecht NE, Hubbard GB, et al. Indocyanine green effect on cultured human retinal pigment epithelial cells: implication for macular hole surgery. Am J Ophthalmol. 2001; 132:433–5.

33. Enaida H, Sakamoto T, Hisatomi T, et al. Morphological and functional damage of the retina caused by intravitreous indocyanine green in rat eyes. Graefes Arch Clin Exp Ophthalmol. 2002; 240:209–13.

34. Yam HF, Kwok AK, Chan KP, et al. Effect of indocyanine green and illumination on gene expression in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2003; 44:370–7.

35. Engelbrecht NE, Freeman J, Sternberg P Jr, et al. Retinal pigment epithelial changes after macular hole surgery with indocyanine green-assisted internal limiting membrane peeling. Am J Ophthalmol. 2002; 133:89–94.

36. Stalmans P, Van Aken EH, Veckeneer M, et al. Toxic effect of indocyanine green on retinal pigment epithelium related to osmotic effects of the solvent. Am J Ophthalmol. 2002; 134:282–5.

37. Oh HN, Lee JE, Kim HW, Yun IH. Clinical outcomes of double staining and additional ILM peeling during ERM surgery. Korean J Ophthalmol. 2013; 27:256–60.

38. Choi ES, Choi YR, Yoon HS. Comparison of outcomes of ILM peeling using triamcinolone and indocyanine green during idiopathic macular hole surgery. J Korean Ophthalmol Soc. 2006; 47:1589–96.
39. Hahm IR, Tae KS, Cho SW, et al. The outcomes after indocyanine green-assisted peeling of the internal limiting membrane in macular hole surgery. J Korean Ophthalmol Soc. 2005; 46:1361–7.
40. Shiono A, Kogo J, Klose G, et al. Effects of indocyanine green staining on the recovery of visual acuity and macular morphology after macular hole surgery. Ophthalmologica. 2013; 230:138–43.

41. Choi YH, Park JW, Cho YW. Internal limiting membrane peeling with or without indocyanine green in macular hole surgery. J Korean Ophthalmol Soc. 2005; 46:1342–50.
42. Haritoglou C, Mauell S, Benoit M, et al. Vital dyes increase the rigidity of the internal limiting membrane. Eye (Lond). 2013; 27:1308–15.

43. Cobos E, Arias L, Ruiz-Moreno J, et al. Preoperative study of the inner segment/outer segment junction of photoreceptors by spectral-domain optical coherence tomography as a prognostic factor in patients with epiretinal membranes. Clin Ophthalmol. 2013; 7:1467–70.

Go to : 
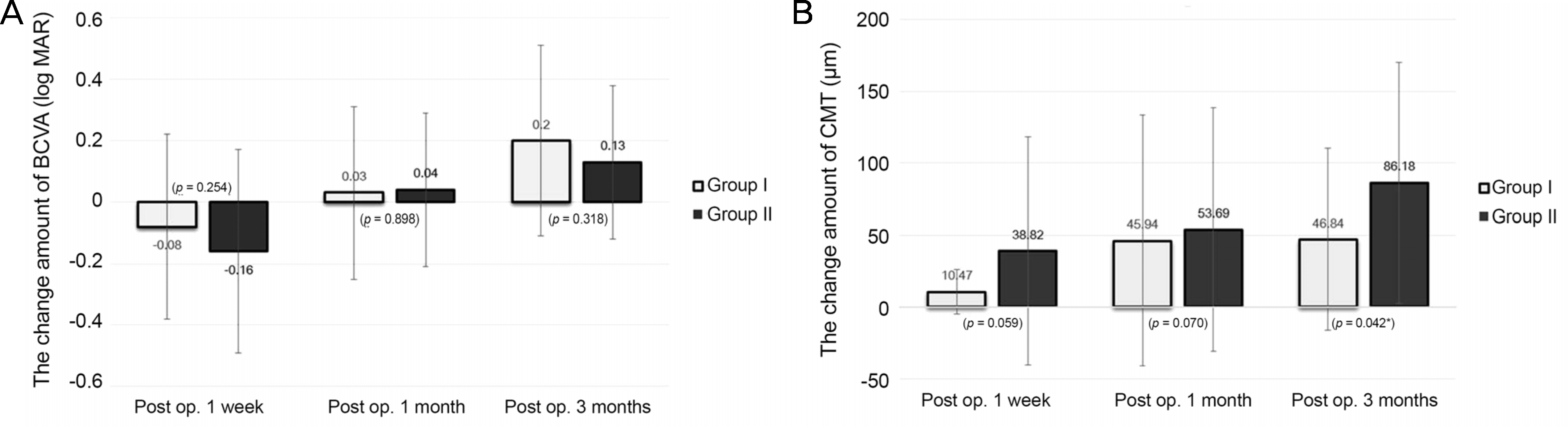 | Figure 1.Comparison of the change amount of BCVA (log MAR) and the CMT (μm) in the two groups. (A) The change amount of BCVA (log MAR) did not differ significantly between the two groups at any point. (B) The change amount of CMT (μm) in the ‘ICG dye with triamcinolone’ group was significantly smaller than that in the ‘triamcinolone only’ group at 3 months after surgery (p = 0.042, *Student t-test). BCVA = best corrected visual acuity; CMT = central macular thickness; Post op. = postoperative; ICG = indocyanine green. |
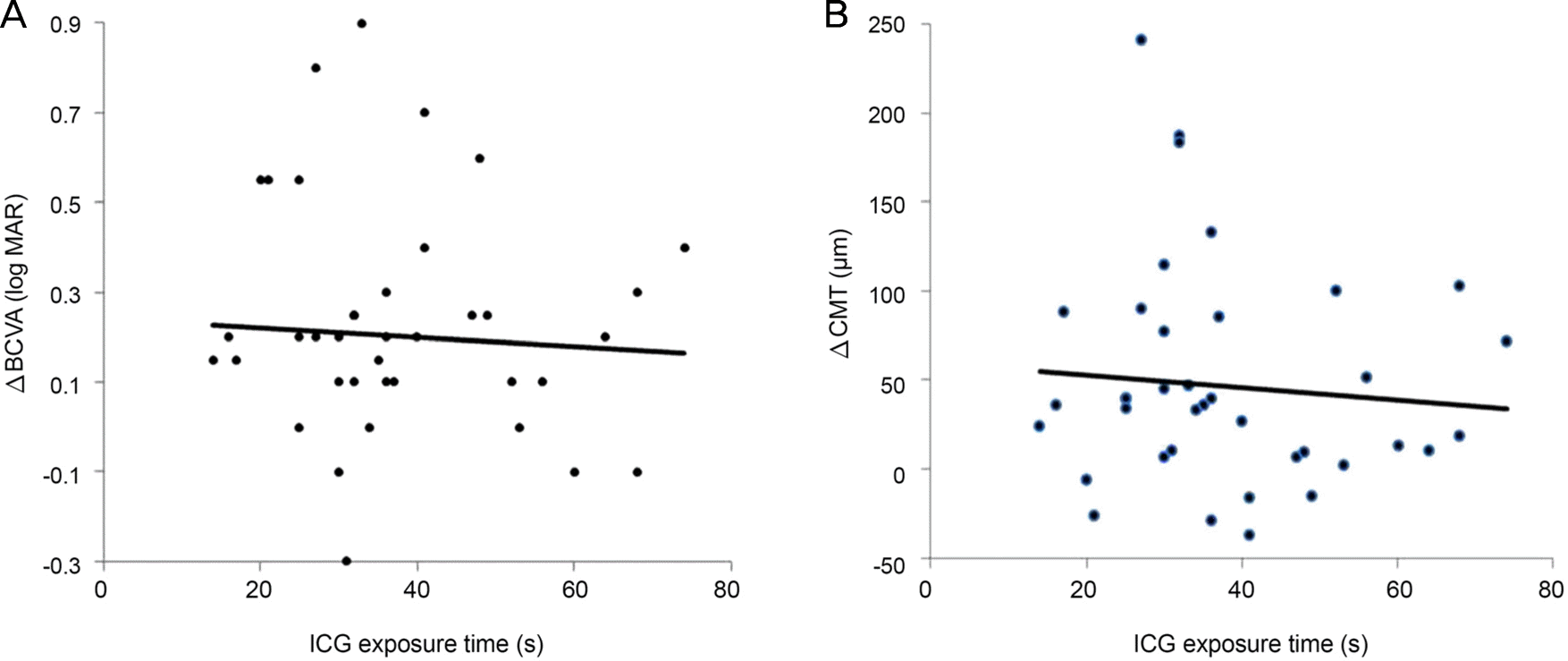 | Figure 2.Scatter plots of ICG exposure time versus BCVA change and CMT change of 3 months value after operation. (A) ICG exposure time is not significantly correlated with BCVA change (r* = −0.55, p = 0.74). (B) ICG exposure time is not significantly correlated with CMT change (r* = −0.312, p = 0.71). ICG = indocyanine green; BCVA = best corrected visual acuity; CMT = central macular thickness. *Pearson's correlation test. |
Table 1.
Comparison of patient's demographics between the two groups
| Group I* (39 eyes) | Group II† (37 eyes) | p-value | |
|---|---|---|---|
| Age (years) | 66.23 ± 9.48 (44-92) | 68.18 ± 8.79 (47-83) | 0.692‡ |
| Male:female | 18:21 | 16:21 | 0.721§ |
| Phakia:pseudophakia | 31:8 | 29:8 | 0.953§ |
| Preop BCVA (log MAR) | 0.55 ± 0.22 | 0.61 ± 0.22 | 0.223† |
| Preop CMT (μm) | 419.69 ± 79.66 | 392.71 ± 109.25 | 0.219‡ |
| Total ICG exposure time (seconds) | 37.74 ± 15.09 (14-74) | (-) | (-) |
| Numbers of ICG injection | 2.33 ± 0.83 (1-4) | (-) | (-) |
Table 2.
Comparison of the BCVA (log MAR) changes after surgery between the two groups
| BCVA (log MAR) | Baseline | Postoperative 1 week | Postoperative 1 month | Postoperative 3 months | p-value‡ |
|---|---|---|---|---|---|
| Group I* | 0.003 | ||||
| BCVA | 0.55 ± 0.22 | 0.66 ± 0.37 | 0.51 ± 0.34 | 0.36 ± 0.36 | |
| Δ | − | −0.08 ± 0.30 | 0.03 ± 0.28 | 0.20 ± 0.31 | |
| Group II† | <0.001 | ||||
| BCVA | 0.61 ± 0.22 | 0.79 ± 0.32 | 0.56 ± 0.24 | 0.48 ± 0.31 | |
| Δ | − | −0.16 ± 0.33 | 0.04 ± 0.25 | 0.13 ± 0.25 | |
| p-value§ | − | 0.254 | 0.898 | 0.318 |
Table 3.
Comparison of the CMT (μm) changes after surgery between the two groups
| CMT (μm) | Baseline | Postoperative 1 week | Postoperative 1 months | Postoperative 3 months | p-value‡ |
|---|---|---|---|---|---|
| Group I* | |||||
| CMT (μm) | 419.69 ± 79.66 | 402.36 ± 64.52 | 386.45 ± 62.16 | 372.71 ± 51.34 | <0.001 |
| Δ | − | 10.47 ± 15.70 | 45.94 ± 87.37 | 46.84 ± 63.32 | |
| Group II† | |||||
| CMT (μm) | 392.71 ± 109.25 | 357.26 ± 78.29 | 342.18 ± 73.66 | 336.97 ± 72.48 | <0.001 |
| Δ | − | 38.82 ± 79.67 | 53.69 ± 84.70 | 86.18 ± 84.04 | |
| p-value§ | − | 0.059 | 0.70 | 0.042 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download