Abstract
Purpose
The purpose of this study was to evaluate the systemic effects of ranibizumab and bevacizumab by examining the plasma levels of anti-vascular endothelial growth factor (anti-VEGF) and VEGF before and after a single intravitreal injection.
Methods
Twenty-eight eyes of 28 patients with various retinal diseases were enrolled. Seventeen eyes received an injection of intravitreal bevacizumab, and 11 eyes received an injection of ranibizumab. Blood samples were collected just before and 1 day, 1 week, and 1 month after injection. Concentrations of anti-VEGF and VEGF in plasma were measured using enzyme-linked immunosorbent assay (ELISA).
Results
In the bevacizumab group, anti-VEGF concentration before the injection was 91.0 ng/mL, while those at 1 day, 1 week, and 1 month post-injection increased to 153.6, 196.3, and 140.3 ng/mL, respectively (p < 0.05 for all). VEGF concentration before the injection was 93.9 pg/mL, while those 1 day, 1 week, and 1 month post-injection were reduced to 40.1, 24.7, and 33.5 pg/mL, respectively (p < 0.05 for all). However, in the ranibizumab group, no significant reductions in anti-VEGF concentration were observed. The anti-VEGF concentration before the injection was 177.6 ng/mL, while those at 1 day, 1 week, and 1 month post-injection were 177.5, 160.7, 175.3 ng/mL, respectively (p > 0.05 for all). VEGF level also showed no significant change. VEGF concentration before the injection was 80.9 pg/mL, while those at 1 day, 1 week and 1 month post-injection were 96.7, 106.3, 106.1 pg/mL, respectively (p > 0.05 for all).
Go to : 
References
1. Tolentino M. Systemic and ocular safety of intravitreal anti-VEGF therapies for ocular neovascular disease. Surv Ophthalmol. 2011; 56:95–113.

2. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004; 25:581–611.

3. Tong JP, Chan WM, Liu DT, et al. Aqueous humor levels of vascular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol. 2006; 141:456–62.

4. Ng EW, Adamis AP. Targeting angiogenesis, the underlying disorder in neovascular age-related macular degeneration. Can J Ophthalmol. 2005; 40:352–68.

5. Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994; 118:445–50.

6. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331:1480–7.

7. Baffert F, Le T, Sennino B, et al. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol. 2006; 290:H547–59.

8. Kamba T, Tam BY, Hashizume H, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006; 290:H560–76.

9. Lee S, Chen TT, Barber CL, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007; 130:691–703.

10. Storkebaum E, Lambrechts D, Carmeliet P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays. 2004; 26:943–54.

11. Blaauwgeers HG, Holtkamp GM, Rutten H, et al. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am J Pathol. 1999; 155:421–8.
12. Marneros AG, Fan J, Yokoyama Y, et al. Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am J Pathol. 2005; 167:1451–9.

13. Nishijima K, Ng YS, Zhong L, et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007; 171:53–67.

14. Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007; 99:1232–9.

15. Gordon MS, Cunningham D. Managing patients treated with bevacizumab combination therapy. Oncology. 2005; 69(Suppl):3:25–33.

16. Bakri SJ, Snyder MR, Reid JM, et al. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology. 2007; 114:2179–82.

17. Gaudreault J, Fei D, Beyer JC, et al. Pharmacokinetics and retinal distribution of ranibizumab, a humanized antibody fragment directed against VEGF-A, following intravitreal administration in rabbits. Retina. 2007; 27:1260–6.

18. Gaudreault J, Fei D, Rusit J, et al. Preclinical pharmacokinetics of ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005; 46:726–33.

19. Xu L, Lu T, Tuomi L, et al. Pharmacokinetics of ranibizumab in patients with neovascular age-related macular degeneration: a population approach. Invest Ophthalmol Vis Sci. 2013; 54:1616–24.

20. Zhang Y, Yao Z, Kaila N, et al. Pharmacokinetics of ranibizumab after intravitreal administration in patients with retinal vein occlusion or diabetic macular edema. Ophthalmology. 2014; 121:2237–46.

21. Avery RL, Castellarin AA, Steinle NC, et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol. 2014; 98:1636–41.

22. Matsuyama K, Ogata N, Matsuoka M, et al. Plasma levels of vascular endothelial growth factor and pigment epithelium-derived factor before and after intravitreal injection of bevacizumab. Br J Ophthalmol. 2010; 94:1215–8.

23. Carneiro AM, Costa R, Falcão MS, et al. Vascular endothelial growth factor plasma levels before and after treatment of neovascular age-related macular degeneration with bevacizumab or ranibizumab. Acta Ophthalmol. 2012; 90:e25–30.

24. Zehetner C, Kirchmair R, Huber S, et al. Plasma levels of vascular endothelial growth factor before and after intravitreal injection of bevacizumab, ranibizumab and pegaptanib in patients with age-related macular degeneration, and in patients with diabetic macular oedema. Br J Ophthalmol. 2013; 97:454–9.

25. Bakri SJ, Snyder MR, Reid JM, et al. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology. 2007; 114:855–9.

26. Rouvas A, Liarakos VS, Theodossiadis P, et al. The effect of intra-vitreal ranibizumab on the fellow untreated eye with subfoveal scarring due to exudative age-related macular degeneration. Ophthalmologica. 2009; 223:383–9.

27. Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006; 113:1695.e1–15.

28. Sawada O, Kawamura H, Kakinoki M, Ohji M. Vascular endothelial growth factor in fellow eyes of eyes injected with intravitreal bevacizumab. Graefes Arch Clin Exp Ophthalmol. 2008; 246:1379–81.

29. Charbel Issa P, Finger RP, Holz FG, Scholl HP. Eighteen-month follow-up of intravitreal bevacizumab in type 2 idiopathic macular telangiectasia. Br J Ophthalmol. 2008; 92:941–5.
30. Al-Dhibi H, Khan AO. Bilateral response following unilateral intravitreal bevacizumab injection in a child with uveitic cystoid macular edema. J AAPOS. 2009; 13:400–2.

31. Velez-Montoya R, Fromow-Guerra J, Burgos O, et al. The effect of unilateral intravitreal bevacizumab (avastin), in the treatment of diffuse bilateral diabetic macular edema: a pilot study. Retina. 2009; 29:20–6.
32. Gamulescu MA, Helbig H. Lack of therapeutic effect of ranibizumab in fellow eyes after intravitreal administration. J Ocul Pharmacol Ther. 2010; 26:213–6.

33. IVAN Study Investigators, Chakravarthy U, Harding SP, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012; 119:1399–411.
34. CATT Research Group, Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011; 364:1897–908.

35. Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group, Martin DF, Maguire MG et al., Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012; 119:1388–98.
Go to : 
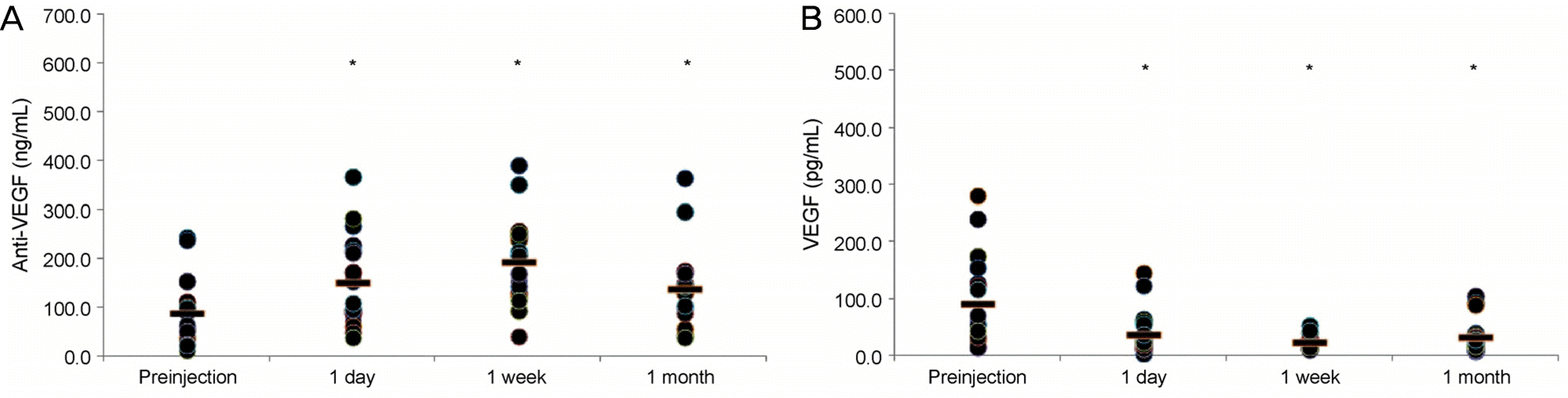 | Figure 1.Plasma levels of anti-vascular endothelial growth factor (VEGF) and VEGF before and after an intravitreal injection of bevacizumab. (A) Anti-VEGF concentrations before and 1 day, 1 week, and 1 month after injection were; 91.0, 153.6, 196.3, and 140.3 ng/mL (p = 0.001, 0.000, 0.001, respectively).(B) VEGF concentrations before and 1 day, 1 week, and 1 month after injection were 93.9, 40.1, 24.7, 33.5 pg/mL (p = 0.006, 0.001, 0.002, respectively). *p < 0.05. |
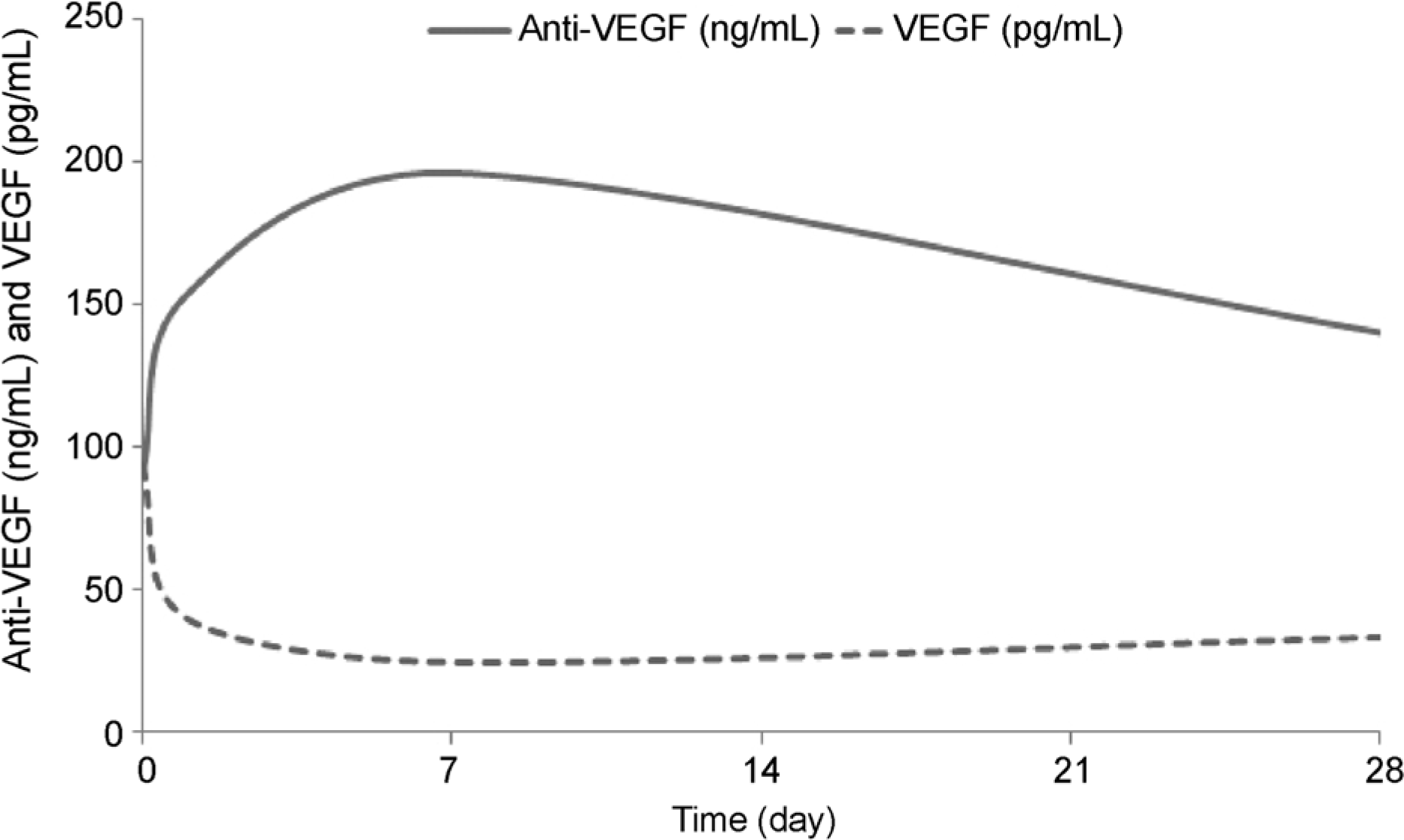 | Figure 2.Schematic graphs of plasma levels of anti-vascular endothelial growth factor (VEGF) and VEGF before and over 1 month after an intravitreal injection of bevacizumab. As anti-VEGF level increased, VEGF level decreased. |
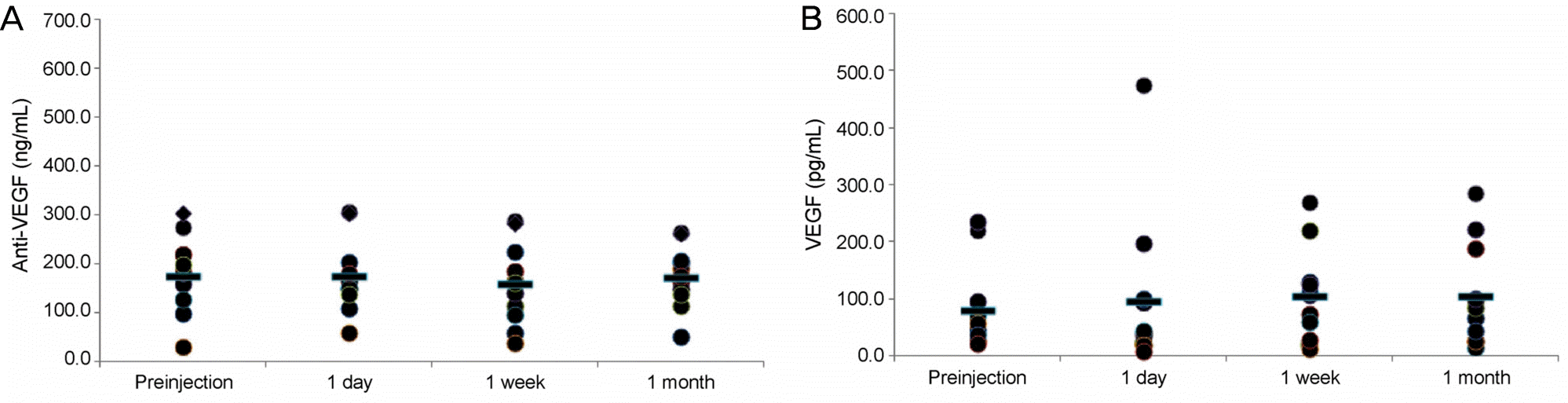 | Figure 3.Plasma levels of anti-vascular endothelial growth factor (VEGF) and VEGF before and after an intravitreal injection of ranibizumab. (A) Anti-VEGF concentrations before injection and 1 day, 1 week, and 1 month after injection were 177.6, 177.5, 160.7, and 175.3 ng/mL (p = 0.646, 0.110, 0.477, respectively). (B) VEGF concentrations before injection and 1 day, 1 week, and 1 month after injection were 80.9, 96.7, 106.3, and 106.1 pg/mL (p = 0.504, 0.328, 0.203, respectively). |
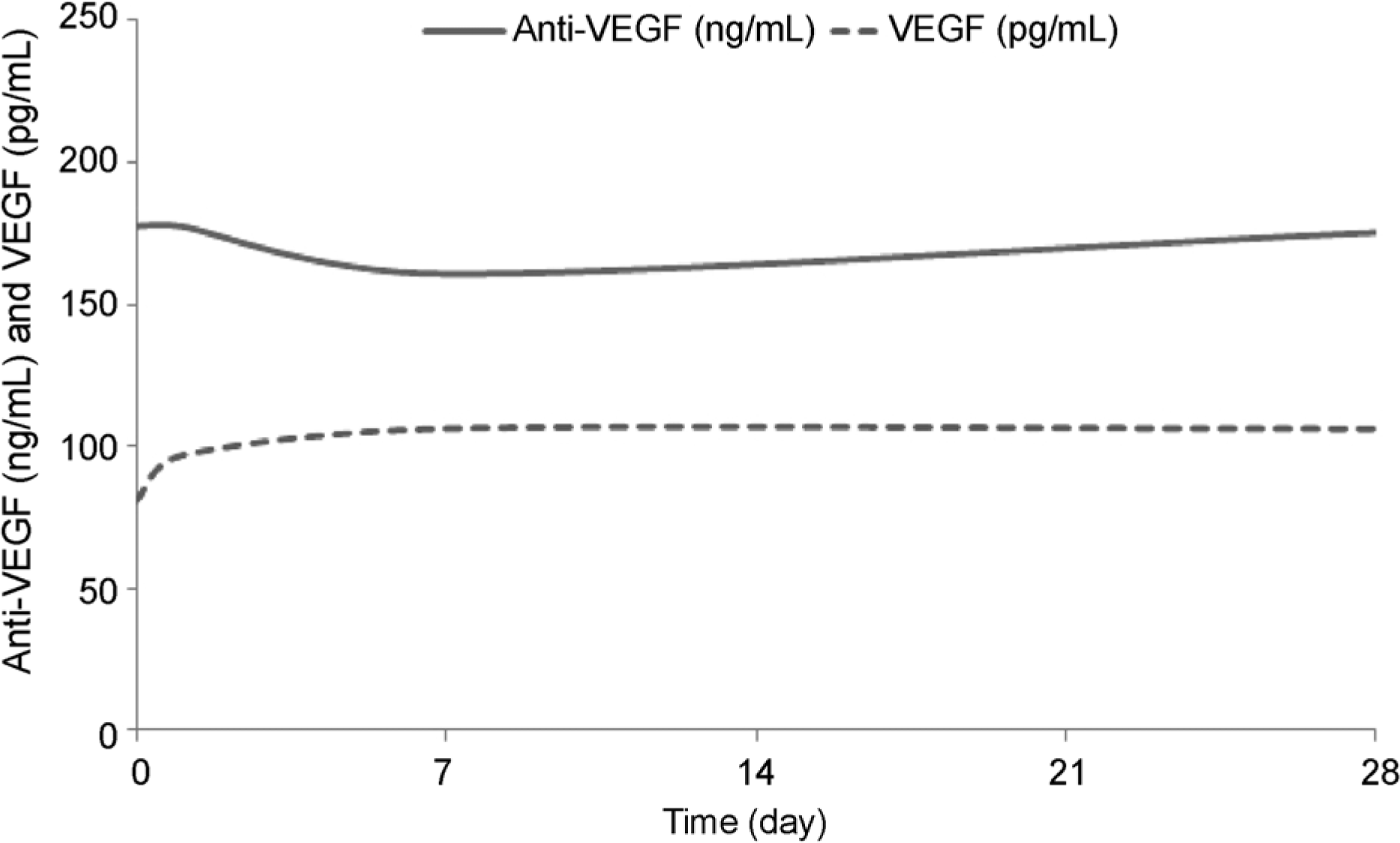 | Figure 4.Schematic graphs of plasma levels of anti-vascular endothelial growth factor (VEGF) and VEGF before and for one month after an intravitreal injection of ranibizumab. No significant change is observed in anti-VEGF or VEGF levels. |
Table 1.
Demographic data
VEGF = vascular endothelial growth factor; M = male; F = female; CNV = choroidal neovascularization; d/t = due to; AMD = age-related macular degeneration; OS = oculus sinister; OD = oculus dexter; DR = diabetic retinopathy; CRVO = central retinal vein occlusion; BRVO = branch retinal vein occlusion; PDR = proliferative diabetic retinopathy.
Table 2.
Plasma concentrations of anti-VEGF and VEGF in the bevacizumab group
| N | Mean | SD |
Percentile |
p-value* | ||
|---|---|---|---|---|---|---|
| 25th | 75th | |||||
| Anti-VEGF | ||||||
| Before | 17 | 91.0 | 67.3 | 40.2 | 107.6 | |
| After 1 day | 17 | 153.6 | 94.4 | 82.0 | 221.6 | 0.001 |
| After 1 week | 17 | 196.3 | 89.7 | 127.1 | 249.1 | 0.000 |
| After 1 month | 17 | 140.3 | 82.8 | 93.5 | 159.7 | 0.001 |
| VEGF | ||||||
| Before | 17 | 93.9 | 80.0 | 29.8 | 140.8 | |
| After 1 day | 17 | 40.1 | 39.6 | 13.4 | 57.2 | 0.006 |
| After 1 week | 17 | 24.7 | 12.4 | 13.4 | 31.4 | 0.001 |
| After 1 month | 17 | 33.5 | 31.9 | 10.6 | 38.8 | 0.002 |
Table 3.
Plasma concentrations of anti-VEGF and VEGF in the ranibizumab group
| N | Mean | SD |
Percentile |
p-value* | ||
|---|---|---|---|---|---|---|
| 25th | 75th | |||||
| Anti-VEGF | ||||||
| Before | 11 | 177.6 | 76.5 | 129.1 | 220.8 | |
| After 1 day | 11 | 177.5 | 73.6 | 139.8 | 205.4 | 0.646 |
| After 1 week | 11 | 160.7 | 82.5 | 97.6 | 225.5 | 0.110 |
| After 1 month | 11 | 175.3 | 62.2 | 139.2 | 207.4 | 0.477 |
| VEGF | ||||||
| Before | 11 | 80.9 | 76.4 | 30.5 | 97.1 | |
| After 1 day | 11 | 96.7 | 137.6 | 21.0 | 102.2 | 0.504 |
| After 1 week | 11 | 106.3 | 82.1 | 28.4 | 131.9 | 0.328 |
| After 1 month | 11 | 106.1 | 89.0 | 27.1 | 190.7 | 0.203 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download