Abstract
Purpose
To evaluate the 6-month outcomes of intravitreal ranibizumab and aflibercept treatment for patients with retinal angiomatous proliferation (RAP).
Methods
A retrospective review of medical records of 28 patients (31 eyes) diagnosed with RAP was performed. All patients were initially treated with 3 consecutive intravitreal ranibizumab or aflibercept injections after diagnosis. Additional treatment was performed when exudation recurred. The best-corrected visual acuity (BCVA) and central foveal thickness were measured before the first injection and 3 and 6 months after the first injection. The values measured before the treatment were compared with those after treatment.
Results
Sixteen eyes were treated with ranibizumab and 15 eyes with aflibercept. The logarithm of minimal angle of resolution (log MAR) values of BCVA before the first injection and 3 and 6 months after the first injection were 0.78 ± 0.50, 0.47 ± 0.30 and 0.59 ± 0.41 in the ranibizumab group and 0.96 ± 0.52, 0.83 ± 0.52 and 0.74 ± 0.56 in the aflibercept group, respectively. Central foveal thickness was 315.75 ± 115.44, 188.38 ± 57.33 and 218.50 ± 96.49 μm in the ranibizumab group and 249.00 ± 74.88, 143.73 ± 32.73 and 196.73 ± 94.08 μm in the aflibercept group, respectively. BCVA was significantly improved and central foveal thickness was significantly reduced at 6 months (p < 0.05) compared to measurements before the first injection in both groups. However, BCVA improvement and central foveal thickness were not significantly different between the 2 groups.
Go to : 
REFERENCES
1). Yannuzzi LA, Negrão S, Iida T, et al. Retinal angiomatous proliferation in age-related macular degeneration. Retina. 2001; 21:416–34.

2). Liu Y, Wen F, Huang S, et al. Subtype lesions of neovascular age-related macular degeneration in Chinese patients. Graefes Arch Clin Exp Ophthalmol. 2007; 245:1441–5.

3). Maruko I, Iida T, Saito M, et al. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol. 2007; 144:15–22.

4). Massacesi AL, Sacchi L, Bergamini F, Bottoni F. The prevalence of retinal angiomatous proliferation in age-related macular degeneration with occult choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2008; 246:89–92.

5). Reche-Frutos J, Calvo-Gonzalez C, Donate-Lopez J, et al. Retinal angiomatous proliferation reactivation 6 months after high-dose intravitreal acetonide triamcinolone and photodynamic therapy. Eur J Ophthalmol. 2007; 17:979–82.

6). Saito M, Shiragami C, Shiraga F, et al. Comparison of intravitreal triamcinolone acetonide with photodynamic therapy and intravitreal bevacizumab with photodynamic therapy for retinal angiomatous proliferation. Am J Ophthalmol. 2010; 149:472–81. e1.

7). Krebs I, Krepler K, Stolba U, et al. Retinal angiomatous proliferation: combined therapy of intravitreal triamcinolone acetonide and PDT versus PDT alone. Graefes Arch Clin Exp Ophthalmol. 2008; 246:237–43.

8). Cho HJ, Lee TG, Han SY, et al. Long-term visual outcome and prognostic factors of Intravitreal anti-vascular endothelial growth factor treatment for retinal angiomatous proliferation. Graefes Arch Clin Exp Ophthalmol. 2015; Apr. 1. [Epub ahead of print].

9). Gharbiya M, Parisi F, Cruciani F, et al. Intravitreal anti-vascular endothelial growth factor for retinal angiomatous proliferation in treatment-naive eyes: long-term functional and anatomical results using a modified PrONTO-style regimen. Retina. 2014; 34:298–305.
10). Kim DB, Kim JH, Jeong SH, et al. Twelve-month outcomes of intravitreal anti-vascular endothelial growth factor therapy for retinal angiomatous proliferation. J Korean Ophthalmol Soc. 2013; 54:1700–7.

11). Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012; 119:2537–48.

12). Bakall B, Folk JC, Boldt HC, et al. Aflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumab. Am J Ophthalmol. 2013; 156:15–22. e1.

13). Cho H, Shah CP, Weber M, Heier JS. Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J Ophthalmol. 2013; 97:1032–5.

14). Yonekawa Y, Andreoli C, Miller JB, et al. Conversion to aflibercept for chronic refractory or recurrent neovascular age-related macular degeneration. Am J Ophthalmol. 2013; 156:29–35. e2.

15). Kawashima Y, Oishi A, Tsujikawa A, et al. Effects of aflibercept for ranibizumab-resistant neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2015; 253:1471–7.

16). Oishi A, Tsujikawa A, Yamashiro K, et al. One-year result of aflibercept treatment on age-related macular degeneration and predictive factors for visual outcome. Am J Ophthalmol. 2015; 159:853–60. e1.

17). Tsaousis KT, Konidaris VE, Banerjee S, Empeslidis T. Intravitreal aflibercept treatment of retinal angiomatous proliferation: a pilot study and short-term efficacy. Graefes Arch Clin Exp Ophthalmol. 2015; 253:663–5.

18). Riusala AM, Immonen IJ. Predictors of structural findings in old disciform lesions. Am J Ophthalmol. 2004; 138:245–53.

19). Shin JY, Yu HG. Optical coherence tomography-based ranibizumab monotherapy for retinal angiomatous proliferation in Korean patients. Retina. 2014; 34:2359–66.

Go to : 
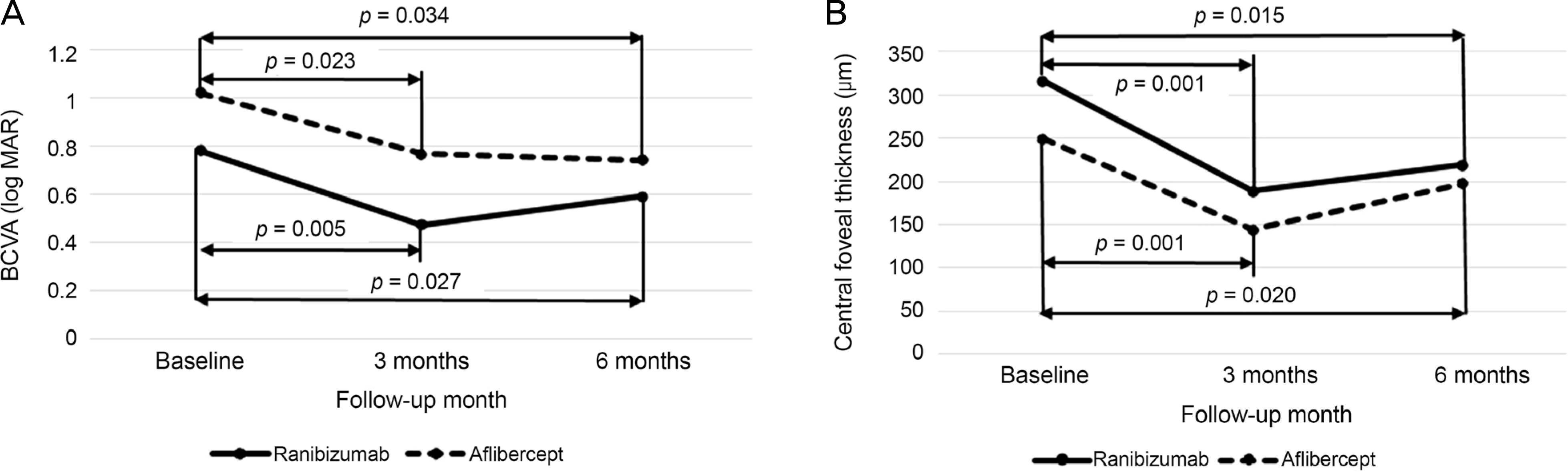 | Figure 1.Six-months changes in BCVA (log MAR) and central foveal thickness in eyes with retinal angiomatous proliferation that were treated with ranibizumab (solid line) or aflibercept (dotted line) treatment, according to the follow-up period. (A) BCVA (log MAR). (B) Central foveal thickness. BCVA = best-corrected visual acuity; log MAR = logarithm of minimal angle of resolution. |
Table 1.
Comparisons of characteristics of 31 included eyes (28 patients) with retinal angiomatous proliferation
| Characteristics | Ranibizumab | Aflibercept | p-value |
|---|---|---|---|
| Age (years) | 75.19 ± 6.36 | 73.47 ± 7.80 | 0.928* |
| Sex (n, %) | 0.843† | ||
| Male | 3 (20.0) | 3 (23.1) | |
| Female | 12 (80.0) | 10 (76.9) | |
| log MAR BCVA before 1st injection | 0.78 ± 0.50 | 0.96 ± 0.52 | 0.338* |
| log MAR BCVA 6 months after 1st injection | 0.59 ± 0.41 | 0.74 ± 0.56 | 0.682* |
| Amount of change in log MAR BCVA during 6 months | −0.19 ± 0.31 | −0.10 ± 0.61 | 0.770* |
| CRT before 1st injection (μ m) | 315.75 ± 115.44 | 249.00 ± 74.88 | 0.078* |
| CRT 6 months after 1st injection (μ m) | 218.50 ± 96.49 | 196.73 ± 94.08 | 0.520* |
| Amount of change in CRT during 6 months (μ m) | −97.25 ± 125.73 | −52.27 ± 87.44 | 0.470* |
| Number of anti-VEGF injections | 3.50 ± 0.63 | 3.20 ± 0.56 | 0.188* |
| Number of eyes required 4th injection (%) | 7 (43.75) | 2 (13.3) | 0.062† |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download