Abstract
Purpose
In the present study, 1-year outcome of intravitreal dexamethasone implant in macular edema secondary to central retinal vein occlusion (CRVO) was evaluated.
Methods
The medical records of 22 patients (22 eyes) with macular edema secondary to CRVO were reviewed retrospectively. All patients were treated with intravitreal dexamethasone implant more than twice a year and followed up at least for 1 year from the first dexamethasone implant injection. The best-corrected visual acuity (BCVA), central macular thickness (CMT), and intraocular pressure (IOP) were measured every 2 months after the first injection. Adverse effects, including cataract formation and elevation of IOP, were analyzed.
Results
The mean patient age was 64.3 ± 9.5 years and 10 patients (45.5%) were male. The average number of injections was 2.4 ± 0.6 and the interval between the first and second injection was 22.0 ± 6.4 weeks. The mean BCVA (log MAR) was 0.82 ±0.50 and 0.72 ± 0.62 at baseline and after 1 year, respectively. Vision was significantly improved for 8 months after the first injection (p < 0.05). However, vision was not different from baseline after 1 year. The CMT was significantly decreased compared to baseline (p < 0.001). Subgroup analysis revealed that BCVA was improved and CMT decreased significantly when intravitreal dexamethasone concentration was presumed sufficient. Moreover, CMT decreased significantly in hypertensive and ischemic groups compared with normotensive and non-ischemic groups, respectively (p < 0.001). Elevated IOP was observed in 6 eyes (27.3%), but all 6 eyes became normal after topical agent was applied. Cataract formation was observed in 3 eyes (13.6%).
Go to : 
References
1. Klaver CC, Wolfs RC, Vingerling JR, et al. Age-specific prevalence and causes of blindness and visual impairment in an older abdominal: the Rotterdam Study. Arch Ophthalmol. 1998; 116:653–8.
2. Attebo K, Mitchell P, Smith W. Visual acuity and the causes of abdominal loss in Australia. The Blue Mountains Eye Study. Ophthalmology. 1996; 103:357–64.
3. Rehak M, Wiedemann P. Retinal vein thrombosis: pathogenesis and management. J Thromb Haemost. 2010; 8:1886–94.

4. Lee JY, Kim HC. Ganglion cell layer thickness after anti-vascular endothelial growth factor treatment in retinal vein occlusion. J Korean Ophthalmol Soc. 2016; 57:63–70.

5. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. The Central Vein Occlusion Study Group M report. Ophthalmology. 1995; 102:1425–33.
6. Prager F, Michels S, Kriechbaum K, et al. Intravitreal bevacizumab (Avastin) for macular oedema secondary to retinal vein occlusion: 12-month results of a prospective clinical trial. Br J Ophthalmol. 2009; 93:452–6.
7. Zhang H, Liu ZL, Sun P, Gu F. Intravitreal bevacizumab for abdominal of macular edema secondary to central retinal vein occlusion: eighteen-month results of a prospective trial. J Ocul Pharmacol Ther. 2011; 27:615–21.
8. Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010; 117:1124–33.e1.
9. Korobelnik JF, Holz FG, Roider J, et al. Intravitreal aflibercept abdominal for macular edema resulting from central retinal vein abdominal: one-year results of the phase 3 GALILEO Study. Ophthalmology. 2014; 121:202–8.
10. Heier JS, Clark WL, Boyer DS, et al. Intravitreal aflibercept abdominal for macular edema due to central retinal vein occlusion: two-year results from the COPERNICUS Study. Ophthalmology. 2014; 121:1414–20.e1.
11. Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial abdominal the efficacy and safety of intravitreal triamcinolone with abdominal to treat vision loss associated with macular edema abdominal to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009; 127:1101–14.
12. Haller JA, Bandello F, Belfort R Jr, et al. Dexamethasone abdominal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011; 118:2453–60.
13. Dugel PU, Capone A Jr, Singer MA, et al. Two or more abdominal intravitreal implants in treatment-naïve patients with macular edema due to retinal vein occlusion: subgroup analysis of a retrospective chart review study. BMC Ophthalmol. 2015; 15:118.

14. Capone A Jr, Singer MA, Dodwell DG, et al. Efficacy and safety of two or more dexamethasone intravitreal implant injections for treatment of macular edema related to retinal vein occlusion (Shasta Study). Retina. 2014; 34:342–51.

15. Haller JA, Bandello F, Belfort R Jr, et al. Randomized, sham-abdominalled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010; 117:1134–46.e3.
16. Coscas G, Loewenstein A, Augustin A, et al. Management of abdominall vein occlusion–consensus document. Ophthalmologica. 2011; 226:4–28.
17. Sohn HJ, Han DH, Kim IT, et al. Changes in aqueous abdominal of various cytokines after intravitreal triamcinolone versus bevacizumab for diabetic macular edema. Am J Ophthalmol. 2011; 152:686–94.
18. Saraiya NV, Goldstein DA. Dexamethasone for ocular inflammation. Expert Opin Pharmacother. 2011; 12:1127–31.

19. Shim SH, Hah JH, Hwang SY, et al. Dexamethasone treatment abdominal VEGF production via suppression of STAT3 in a head and neck cancer cell line. Oncol Rep. 2010; 23:1139–43.

20. Greenberger S, Boscolo E, Adini I, et al. Corticosteroid abdominal of VEGF-A in infantile hemangioma-derived stem cells. N Engl J Med. 2010; 362:1005–13.
21. Chang-Lin JE, Attar M, Acheampong AA, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone abdominal implant. Invest Ophthalmol Vis Sci. 2011; 52:80–6.
22. Joshi L, Yaganti S, Gemenetzi M, et al. Dexamethasone implants in retinal vein occlusion: 12-month clinical effectiveness using repeat injections as-needed. Br J Ophthalmol. 2013; 97:1040–4.

23. Moisseiev E, Goldstein M, Waisbourd M, et al. abdominal evaluation of patients treated with dexamethasone intravitreal implant for macular edema due to retinal vein occlusion. Eye (Lond). 2013; 27:65–71.
24. Garweg JG, Zandi S. Retinal vein occlusion and the use of a abdominal intravitreal implant (Ozurdex(R)) in its treatment. Graefes Arch Clin Exp Ophthalmol. 2016; 254:1257–65.
Go to : 
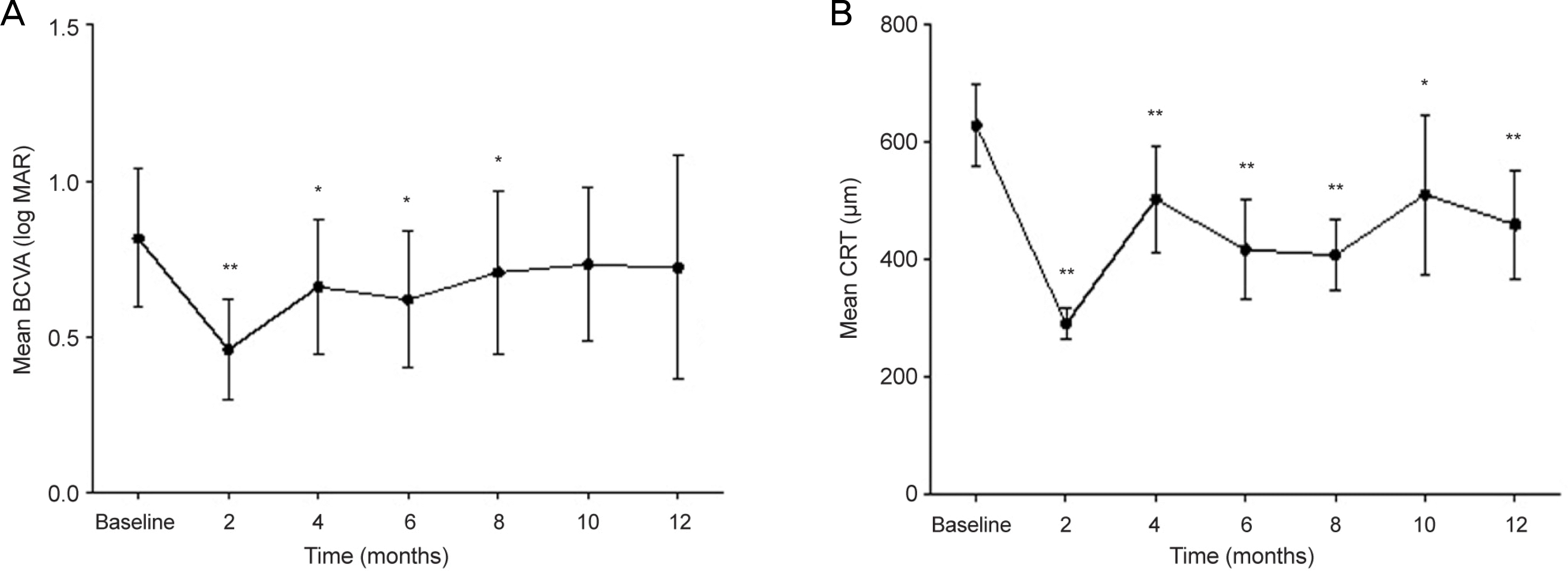 | Figure 1.Best corrected visual acuity (BCVA) and central macular thickness (CMT) results. (A) BCVA was significantly increased at 2, 4, 6, 8 months after first injection. (B) CMT was significantly decreased at 2, 6, 8, 12 months after first injection. * p<0.05, against baseline by paired t-test; ** p<0.01, against baseline by paired t-test. |
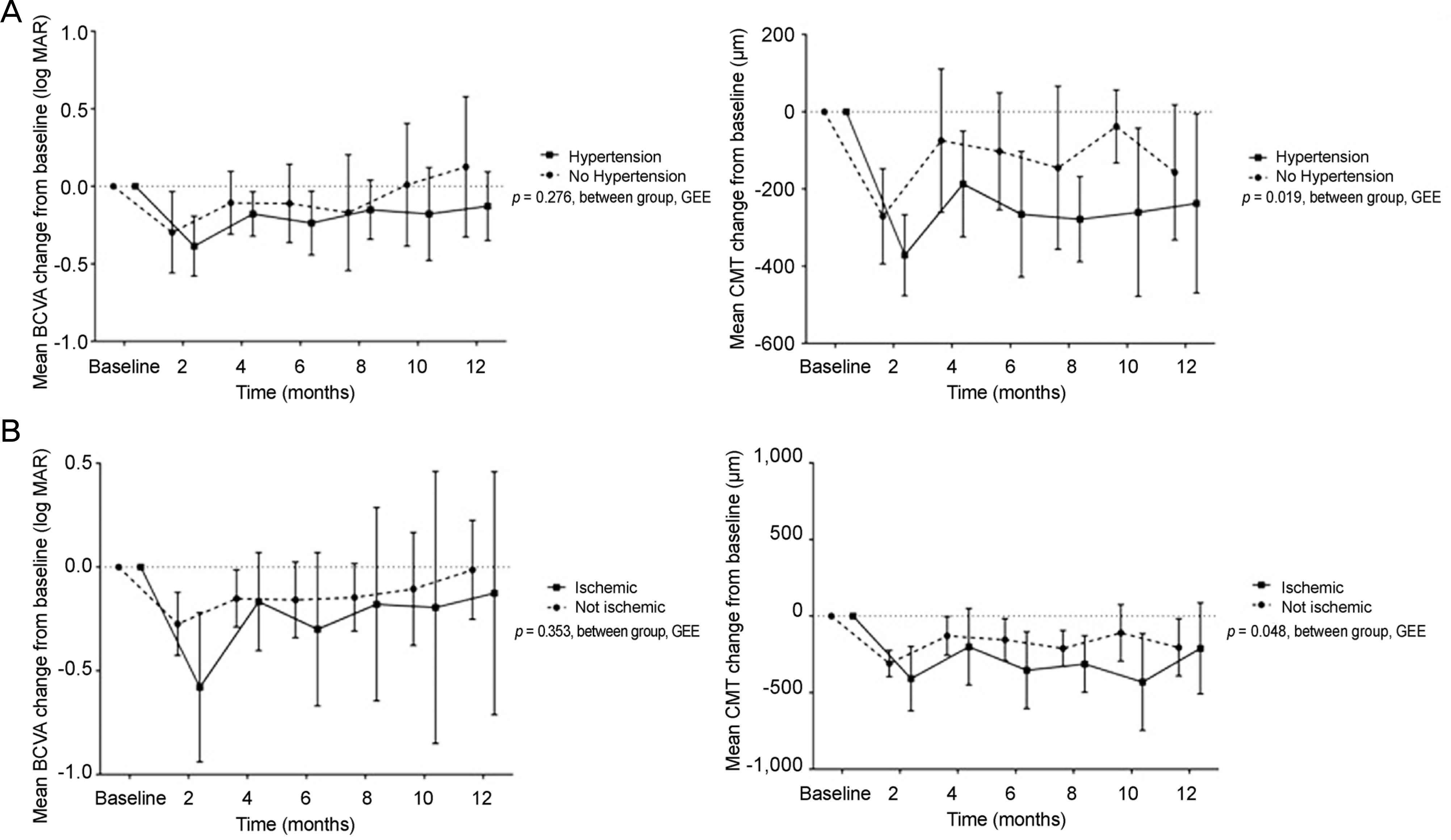 | Figure 2.Univariate analysis (by generalized estimating equation [GEE]) for best corrected visual acuity (BCVA) and central macular thickness (CMT) change from baseline. (A) Hypertension (HTN) group showed no significant difference in BCVA but significantly large decrease in CMT compared tonon-HTN group. (B) Ischemic group neither showed significant difference in BCVA but presented significantly large decrease in CMT compared tonon-HTN group. |
Table 1.
Baseline characteristics of enrolled patients
Table 2.
Adverse effects of ozurdex injection and results about injection number or interval in follow-up period
Table 3.
Multivariate analysis (by GEE) for BCVA change from baseline
| Estimate | B | S.E. |
95% CI |
p-value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| DM vs. No DM | –0.092 | 0.1204 | –0.328 | 0.144 | 0.447 |
| HTN vs. No HTN | –0.090 | 0.0764 | –0.240 | 0.060 | 0.239 |
| Ischemic CRVO vs. Non-ischemic CRVO | –0.013 | 0.1327 | –0.247 | 0.010 | 0.922 |
| Age | –0.007 | 0.0062 | –0.019 | 0.006 | 0.290 |
| ‘High’ concentration section | –0.380 | 0.0793 | –0.536 | –0.225 | 0.000* |
| ‘Slope’ concentration section | –0.417 | 0.0781 | –0.570 | –0.264 | 0.000* |
| ‘Low’ concentration section | –0.313 | 0.0635 | –0.437 | –0.188 | 0.000* |
| Prior Tx. vs. Naive | 0.036 | 0.0924 | –0.145 | 0.218 | 0.693 |
Table 4.
Multivariate analysis (by GEE) for CMT change from baseline
| Estimate | B | S.E. |
95% CI |
p-value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| DM vs. No DM | 86.657 | 53.1710 | –17.556 | 190.870 | 0.103 |
| HTN vs. No HTN | –103.496 | 37.6942 | –177.375 | –29.616 | 0.006* |
| Ischemic CRVO vs. Non-ischemic CRVO | –101.271 | 47.1429 | –193.669 | –8.873 | 0.032* |
| ‘High’ concentration section | –349.848 | 48.5683 | –445.040 | –254.655 | 0.000* |
| ‘Slope’ concentration section | –345.252 | 32.5841 | –409.116 | –281.389 | 0.000* |
| ‘Low’ concentration section | –105.684 | 38.1145 | –180.387 | –30.981 | 0.006* |
| Prior Tx. vs. Naive | –49.371 | 53.7578 | –154.735 | 55.992 | 0.358 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download