Abstract
Purpose
To investigate the difference of optical coherence tomography (OCT) findings between the aflibercept treatment group and the ranibizumab treatment group.
Methods
This study includes patients diagnosed with treatment-naïve neovascular age-related macular degeneration (AMD), and they were treated with aflibercept (n = 23, 23 eyes) or ranibizumab (n = 26, 26 eyes) monthly for 3 months. In this study, the aflibercept treatment group patients were treated from March 2014 to April 2015, and the ranibizumab treatment group patients were treated from December 2008 to April 2015. After three initial injections, they were followed up monthly for an additional 3 months, and additional treatments were performed if necessary. We compared the changes of the two groups before the treatment and after 6 months of treatment, beginning with the OCT findings, such as serous pigment epithelium detachment, fibrovascular pigment epithelium detachment, subretinal fluid, intraretinal fluid, dense zone of outer retina, classic neovascularization, and hyper-reflective dots. We also compared the changes of best corrected visual acuity (BCVA) and inner segment/outer segment (IS/OS) length, external limiting membrane length, and central foveal thickness with optical OCT between the two groups.
Results
In the aflibercept group, 46% of serous epithelial detachments disappeared, while 33% disappeared in the ranibizumab group, and there was significant difference between the two groups (p = 0.01). There was no significant difference in BCVA change or OCT findings between the two groups, but there was a significant difference in serous pigment epithelium detachment.
Conclusions
For treatment of neovascular AMD patients, aflibercept might be more effective in serous pigment epithelium detachment than ranibizumab. Because there was no significant difference in visual acuity improvement in serous pigment detachment for both treatments, it might be necessary to further study the relationship between visual acuity and serous pigment detachment improvement.
References
1. Friedman DS, O'Colmain BJ, Muñoz B, et al. Prevalence of age-abdominal macular degeneration in the United States. Arch Ophthalmol. 2004; 122:564–72.
2. Au Eong KG. Age-related macular degeneration: an emerging abdominal for eye care and public health professionals in the Asia Pacific region. Ann Acad Med Singapore. 2006; 35:133–5.
3. Park KH, Song SJ, Lee WK, et al. The results of abdominal abdominal of age-related macular degeneration in Korea. J Korean Ophthalmol Soc. 2010; 51:516–23.
4. Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002; 99:11393–8.

5. Lommatzsch A, Heimes B, Gutfleisch M, et al. Serous pigment abdominal detachment in age-related macular degeneration: comparison of different treatments. Eye (Lond). 2009; 23:2163–8.
6. Klettner A, Recber M, Roider J. Comparison of the efficacy of abdominal, ranibizumab, and bevacizumab in an RPE/choroid organ culture. Graefes Arch Clin Exp Ophthalmol. 2014; 252:1593–8.
7. Cho H, Shah C, Weber M, Heier JS. Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J Ophthalmol. 2013; 97:1032–5.

8. Bakall B, Folk JC, Boldt HC, et al. Aflibercept therapy for abdominal age-related macular degeneration resistant to bevacizumab and ranibizumab. Am J Ophthalmol. 2013; 156:15–22.e1.
9. Patel KH, Chow CC, Rathod R, et al. Rapid response of retinal abdominal epithelial detachments to intravitreal aflibercept in neovascular age-related macular degeneration refractory to abdominal and ranibizumab. Eye (Lond). 2013; 27:663–7.
10. Kumar N, Marsiglia M, Mrejen S, et al. Visual and anatomical abdominals of intravitreal aflibercept in eyes with persistent subfoveal fluid despite previous treatments with ranibizumab in patients with neovascular age-related macular degeneration. Retina. 2013; 33:1605–12.
11. Böhni SC, Bittner M, Howell JP, et al. Comparison of Eylea(R) with Lucentis(R) as first-line therapy in patients with treatment-naïve neovascular age-related macular degeneration in real-life clinical practice: retrospective case-series analysis. BMC Ophthalmol. 2015; 15:109.

12. Dirani A, Ambresin A, Marchionno L, et al. Factors influencing the treatment response of pigment epithelium detachment in age-rea-lated macular degeneration. Am J Ophthalmol. 2015; 160:732–8.e2.
13. Papadopoulos N, Martin J, Ruan Q, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related li-gands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012; 15:171–85.

14. Cho HJ, Kim KM, Kin HS, et al. Response of pigment epithelial abdominal to anti-vascular endothelial growth factor treatment in age-related macular degeneration. Am J Ophthalmol. 2016; 166:112–9.
15. Hata M, Oishi A, Tsujikawa A, et al. Efficacy of intravitreal abdominal of aflibercept in neovascular age-related macular abdominal with or without choroidal vascular hyperpermeability. Invest Ophthalmol Vis Sci. 2014; 55:7874–80.
Figure 1.
Spectral domain optical coherence tomography images (SD-OCT). (A) Before the treatments, serous pigment epithelium detachment (S-PED); subretinal fluid (SRF); hyper-reflective dots (H-dots) were found on the SD-OCT images. (B) After 6 month of treatment, S-PED and SRF disappeared on the SD-OCT images. (C) Before the treatments, Intraretinal fluid (IRF); Dense Zone of the outer retina (D-zone); classic choroidal neovascularization (C-CNV); hyper-reflective dots (H-dots); fibrovascular pigment epithelium detachment (FV-PED) were found on the SD-OCT images. (D) After 6 months of treatment, IRF, D-zone, C-CNV, H-dots, FV-PED disappeared on the SD-OCT images.
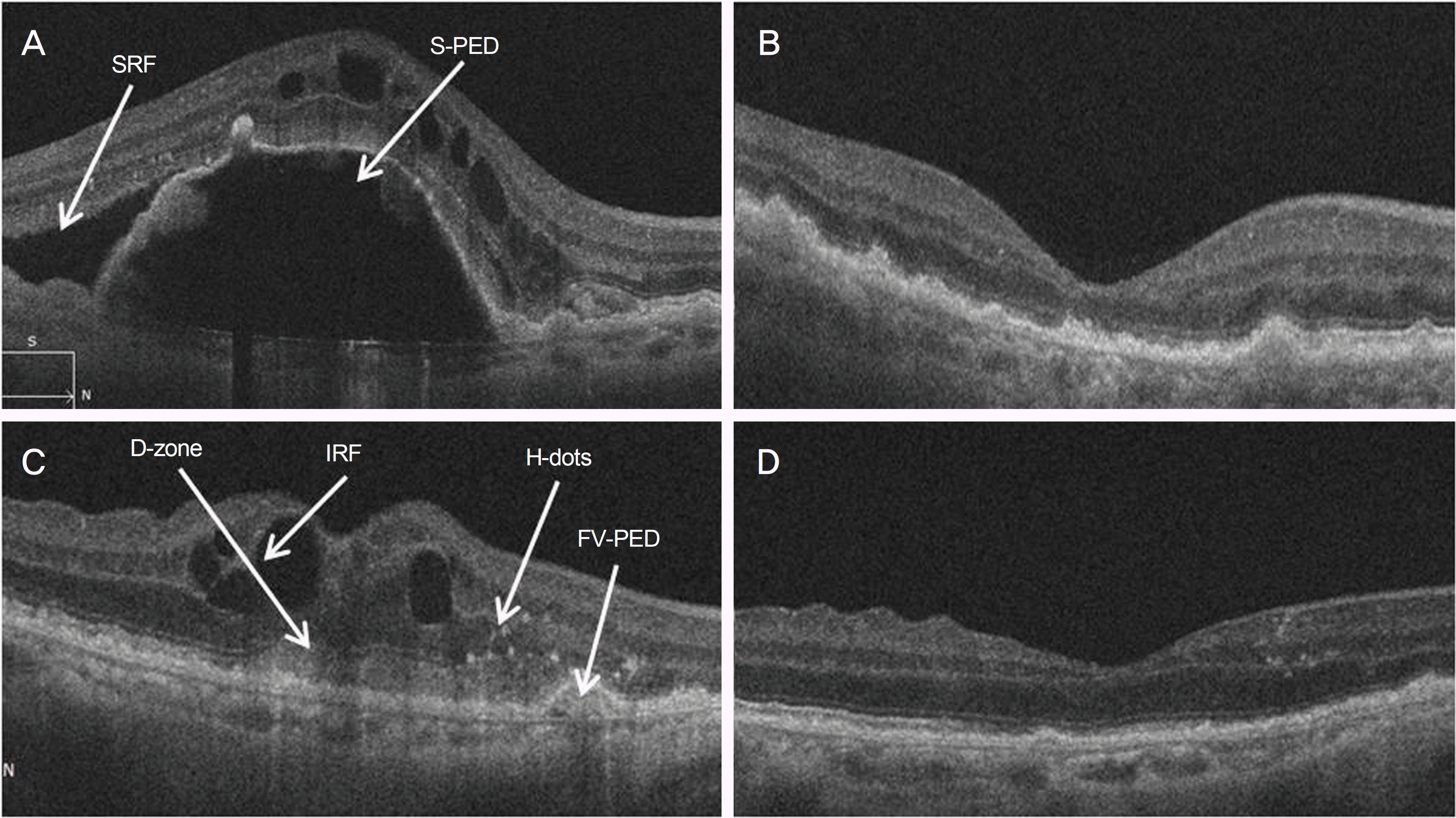
Figure 2.
The inner and outer segment junction (IS/OS) and external limiting membrane (ELM) in Spectral domain optical coherence tomography. These lines were measured within 1 mm from the central fovea. The dashed black arrows indicate the ELM lines, and the dashed white arrows indicate the IS/OS line.
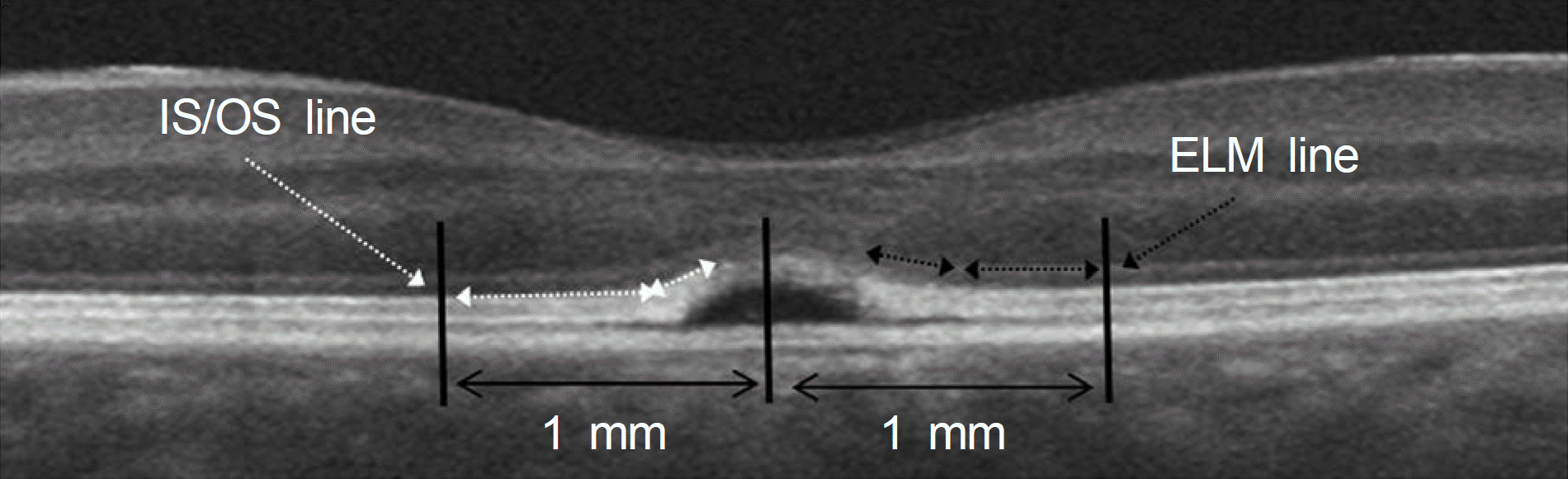
Table 1.
Baseline demographics and ocular characteristics of the two treatment groups
| Aflibercept group | Ranibizumab group | p-value | |
|---|---|---|---|
| Study population (n) | 23 (23 eyes) | 26 (26 eyes) | |
| Gender (male:female) | 12:11 | 16:10 | 0.527* |
| Age (years) | 68.95 ± 8.45 | 68.61 ± 9.88 | 0.880† |
| Mean baseline BCVA (log MAR) | 1.17 ± 0.46 | 0.90 ± 0.41 | 0.101† |
| Intravitreal injections after 3 loading injections (times) | 0.47 ± 0.66 | 0.88 ± 0.86 | 0.346† |
Table 2.
Comparison of best corrected visual acuity between aflibercept group and ranibizumab group
| Initial (log MAR) | Aflibercept group 1.17 ± 0.46 | Ranibizumab group 0.90 ± 0.41 | p-value 0.101* |
|---|---|---|---|
| Post-treatment (log MAR) | 0.94 ± 0.48 | 0.74 ± 0.39 | 0.222* |
| p-value (log MAR) | 0.763* | 0.289* |
Table 3.
Comparison of optical coherence tomography (OCT) findings between aflibercept group and ranibizumab group
| Aflibercept group | Ranibizumab group | p-value | ||
|---|---|---|---|---|
| S-PED (n, %) | Initial | 15/23 (47) | 6/26 (23) | 0.127* |
| Post-treatment | 8/23 (34) | 4/26 (15) | 0.041* | |
| Improvement | 7/15 (46) | 2/6 (33) | 0.010* | |
| FV-PED (n, %) | Initial | 9/23 (39) | 14/26 (53) | 0.581* |
| Post-treatment | 3/23 (13) | 8/26 (30) | 0.102* | |
| Improvement | 6/9 (66) | 4/14 (28) | 0.102* | |
| C-CNV (n, %) | Initial | 11/23 (47) | 23/26 (88) | 0.152* |
| Post-treatment | 3/23 (13) | 8/26 (30) | 1.000* | |
| Improvement | 8/11 (72) | 15/23 (65) | 1.000* | |
| SRF (n, %) | Initial | 20/23 (86) | 24/26 (92) | 0.126* |
| Post-treatment | 6/23 (26) | 8/26 (30) | 0.537* | |
| Improvement | 14/20 (70) | 16/24 (66) | 1.000* | |
| IRF (n, %) | Initial | 20/23 (86) | 24/26 (92) | 0.126* |
| Post-treatment | 6/23 (24) | 8/26 (30) | 0.627* | |
| Improvement | 14/20 (70) | 16/24 (66) | 0.537* | |
| D-zone (n, %) | Initial | 8/23 (34) | 24/26 (92) | 0.069* |
| Post-treatment | 4/23 (17) | 0/26 (0) | 0.487* | |
| Improvement | 4/8 (50) | 24/24 (100) | 0.487* | |
| H-dot (n, %) | Initial | 11/23 (47) | 14/26 (53) | 0.418* |
| Post-treatment | 8/23 (34) | 9/26 (34) | 1.000* | |
| Improvement | 3/11 (27) | 5/19 (26) | 1.000* | |
| IS/OS length (μ m) | Initial length | 465.78 ± 532.23 | 565.65 ± 583.93 | 0.429† |
| Post-treatment length | 986.69 ± 696.02 | 920.76 ± 749.51 | 0.856† | |
| Change | 506.26 ± 712.71 | 332.15 ± 585.80 | 0.094† | |
| ELM length (μ m) | Initial length | 815.82 ± 683.91 | 1,033.65 ± 678.51 | 0.274† |
| Post-treatment length | 1,181.34 ± 767.31 | 1,435.88 ± 695.58 | 0.316† | |
| Change | 342.34 ± 693.18 | 389.03 ± 776.45 | 0.952† | |
| CFT (μ m) | Initial length | 311.00 ± 104.06 | 312.88 ± 82.31 | 0.904† |
| Post-treatment length | 223.56 ± 68.40 | 234.37 ± 53.65 | 0.489† | |
| Change | –87.34 ± 95.24 | –78.50 ± 74.74 | 0.555† |
Values are presented as mean ± SD unless otherwise indicated. S-PED = serous pigment epithelium detachment; FV-PED = fibrovascular pigment epithelium detachment; C-CNV = classic choroidal neovascularization; SRF = subretinal fluid; IRF = intraretinal fluid; D-zone = dense zone of the outer retina; H-dot = hyper-reflective dots; IS/OS length = length of the intact inner and outer segment junction; ELM length = length of the intact external limiting membrane; CFT = central foveal thickness.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download