Abstract
Purpose
To investigate the clinical features and therapeutic effects of unilateral ptosis in patients who respond to the phenylephrine (PE) test.
Methods
Patients who presented with unilateral ptosis from January 2010 to December 2014 and underwent a PE test were included in the analysis. A 2.5% ophthalmic solution of phenylephrine hydrochloride was instilled at the superior conjunctival fornix in the ptotic eye. After 10 minutes of instillation, the patients' eyelid heights were evaluated. Underlying systemic diseases were examined based on previous medical history, a blood test, neurologic examination, and radiologic imaging findings.
Results
Twenty-six of 44 patients who underwent a PE test showed positive results. Fourteen (53.8%) patients with positive PE test had systemic disease, and 2 (11.2%) patients had systemic disease with negative PE test. In the positive PE test group, the associated systemic disease frequency was remarkably high (p = 0.004). Myasthenia gravis (MG) was found more frequently in the positive PE test group than in the negative PE test group (p = 0.031). After 6 months, the interpalpebral fissure height increased by 2.20 mm in the positive PE test group and 2.38 mm in the negative PE test group. Patients receiving medication treatment experienced an increase in interpalpebral fissure height of 2.00 mm in the positive PE test group and 2.50 mm in the negative PE test group. In patients undergoing observation alone, the interpalpebral fissure height increased by 1.50 mm in the positive PE test group and 0.80 mm in the negative PE test group. There was no significant difference in treatment methods (respectively, p = 0.147, p = 0.228 and p = 0.112).
Go to : 
References
1. Finsterer J. Ptosis: causes, presentation, and management. Aesthetic Plast Surg. 2003; 27:193–204.

3. Beard C. Ptosis. 3rd ed.St. Louis: Mosby;1981. p. 39–75.
4. Tarbet KJ, Lemke BN. Anatomy of the eyelids and lacrimal abdominal system. Albert DM, Jakobiec FA, editors. Principles and Practice of Ophthalmology. 3rd ed.Philadelphia: WB Saunders;2008. chap. 243.
5. Kim IS, Choi JB, Rah SH, Lee SY. Classification of ptosis in Korea. J Korean Ophthalmol Soc. 2005; 46:1262–9.
6. Dresner SC. Further modifications of the Müller's muscle-abdominal resection procedure for blepharoptosis. Ophthal Plast Reconstr Surg. 1991; 7:114–22.
7. Bae JS, Ha MS, Lee JY, et al. Results of conjunctiva-Muller muscle resection in mild eyelid ptosis. J Korean Ophthalmol Soc. 2008; 49:1365–70.

8. Lyon DB, Gonnering RS, Dortzbach RK, Lemke BN. Unilateral ptosis and eye dominance. Ophthal Plast Reconstr Surg. 1993; 9:237–40.

9. Dresner SC. Ptosis management: a practical approach. Chen WP, editor. Oculoplastic surgery: the essentials. 1st ed.New York: Thieme Medical Publishers;2001. chap. 6.
10. Park DI, Ha SW, Lew H. Clinical outcomes of conjunctiva-Muller muscle resection and factors which affect success. J Korean Ophthalmol Soc. 2011; 52:1263–8.
11. Oosterhuis HJ. The ocular signs and symptoms of myasthenia gravis. Doc Ophthalmol. 1982; 52:363–78.

12. Skibell BC, Harvey JH, Oestreicher JH, et al. Adrenergic receptors in the ptotic human eyelid: correlation with phenylephrine testing and surgical success in ptosis repair. Ophthal Plast Reconstr Surg. 2007; 23:367–71.

13. Esmaeli-Gutstein B, Hewlett BR, Pashby RC, et al. Distribution of adrenergic receptor subtypes in the retractor muscles of the upper eyelid. Ophthal Plast Reconstr Surg. 1999; 15:92–9.

14. Wikberg-Matsson A, Uhlén S, Wikberg JE. Characterization of abdominal(1)-adrenoceptor subtypes in the eye. Exp Eye Res. 2000; 70:51–60.
15. Huang Y, Gil DW, Vanscheeuwijck P, et al. Localization of alpha 2-adrenergic receptor subtypes in the anterior segment of the abdominal eye with selective antibodies. Invest Ophthalmol Vis Sci. 1995; 36:2729–39.
16. Oosterhuis HJ. The natural course of myasthenia gravis: a long term follow up study. J Neurol Neurosurg Psychiatry. 1989; 52:1121–7.

17. Nagane Y, Utsugisawa K, Suzuki S, et al. Topical naphazoline in the treatment of myasthenic blepharoptosis. Muscle Nerve. 2011; 44:41–4.

18. Bradley EA, Bartley GB, Chapman KL, Waller RR. Surgical abdominal of blepharoptosis in patients with myasthenia gravis. Ophthal Plast Reconstr Surg. 2001; 17:103–10.
19. Lai CS, Lai YW, Huang SH, et al. Surgical correction of the abdominal blepharoptosis in patients with ocular myasthenia gravis. Ann Plast Surg. 2016; 76(Suppl 1):S55–9.
Go to : 
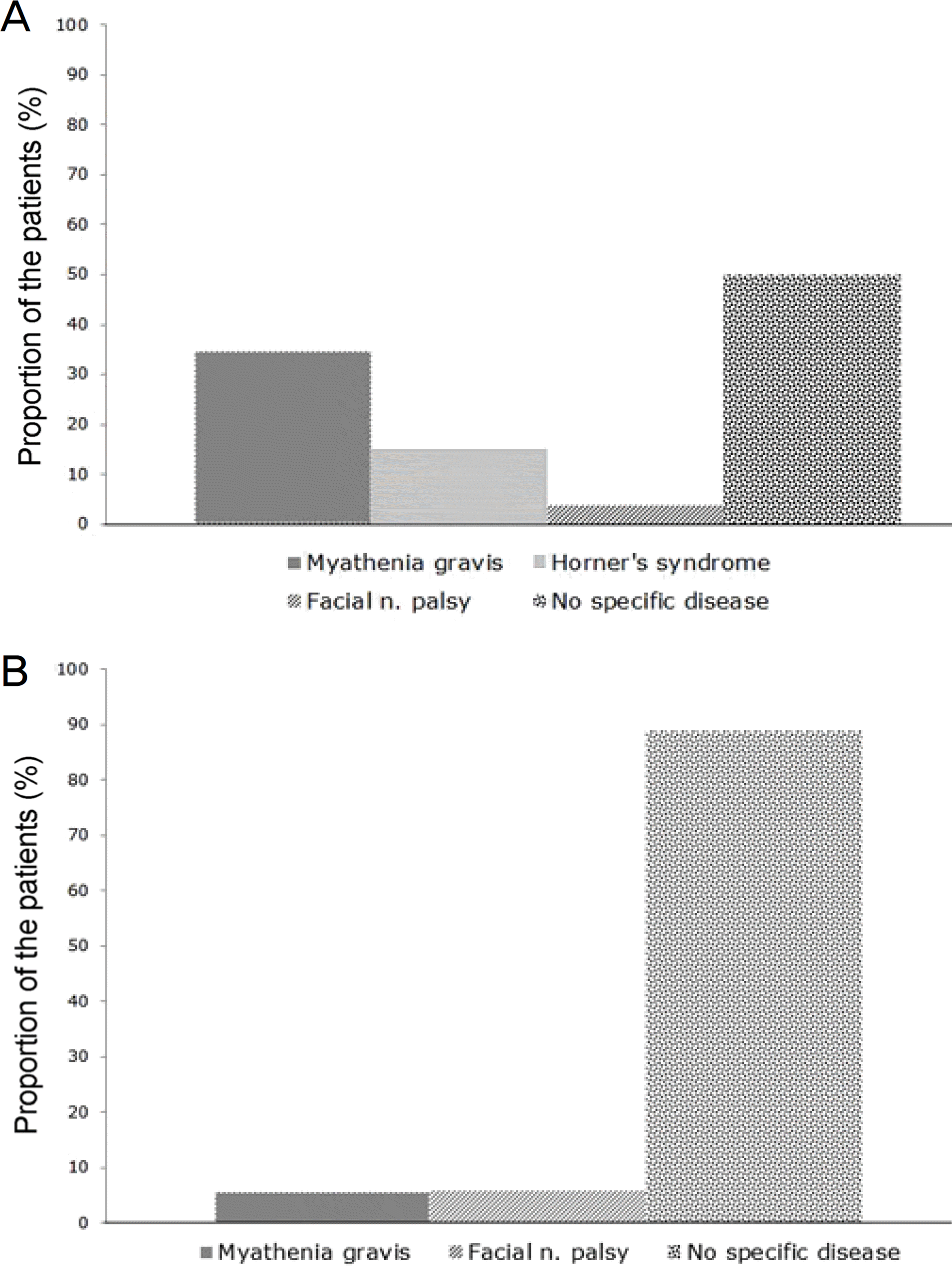 | Figure 1.Distribution of associated systemic diseases in patients with unilateral ptosis according to phenylephrine test results. (A) Positive, (B) Negative. The systemic disease was associated more frequently in patients with positive results. |
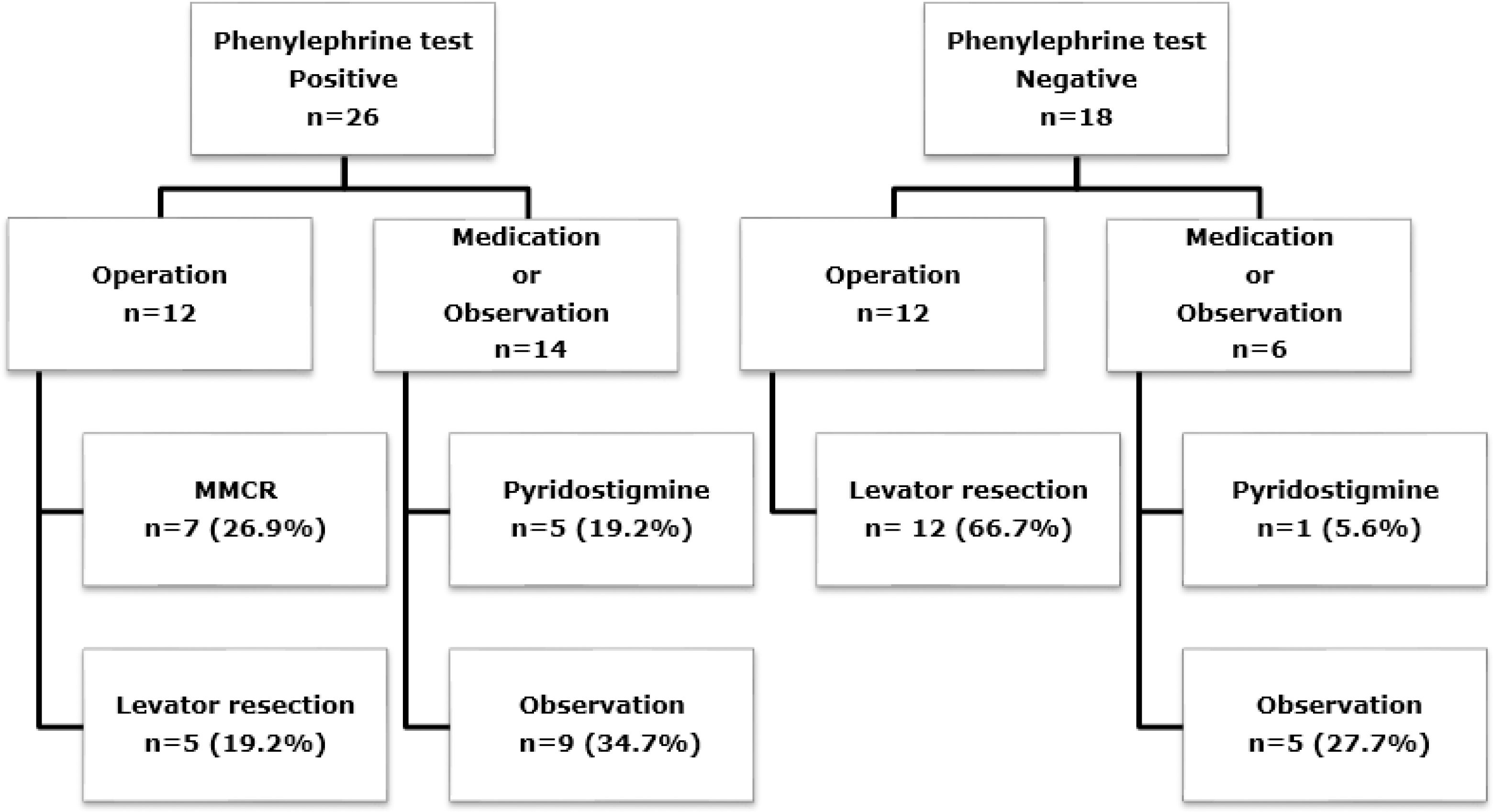 | Figure 2.Distribution of patients according to the management of unilateral ptosis with positive or negative phenylephrine test result. 12 of 26 (46.1%) patients with positive results and 12 of 18 (66.7%) patients with negative results underwent operation. The remaining patients were treated with medication or observed. MMCR = Müller's muscle conjunctival resection. |
Table 1.
Characteristics of patients
Table 2.
Change of Interpalpebral fissure heights (mm) before and after treatment
| Treatment method |
Before treatment (mm) |
6 months after treatment (mm) |
Difference (mm) |
|||
|---|---|---|---|---|---|---|
| PE | test | PE | test | PE | test | |
| Positive | Negative | Positive | Negative | Positive | Negative | |
| Operation | 5.54 ± 1.16 | 4.56 ± 1.53 | 7.75 ± 1.21 | 7.00 ± 0.94 | 2.20 ± 0.78 | 2.38 ± 1.43 |
| MMCR | 5.50 ± 1.29 | – | 7.85 ± 0.99 | – | 2.36 ± 0.63 | – |
| Levator resection | 5.60 ± 1.08 | 4.56 ± 1.53 | 7.60 ± 1.59 | 7.00 ± 0.94 | 2.00 ± 1.00 | 2.38 ± 1.43 |
| Medication | 5.00 ± 0.00 | 3.00 ± 0.00 | 7.00 ± 1.77 | 5.50 ± 0.00 | 2.00 ± 1.78 | 2.50 ± 0.00 |
| Observation | 5.70 ± 1.25 | 6.00 ± 2.32 | 7.20 ± 1.34 | 6.80 ± 2.13 | 1.50 ± 0.70 | 0.80 ± 1.62 |
| p-value* | 0.521 | 0.462 | 0.379 | 0.154 | 0.446 | 0.168 |
| p-value† | 0.539 | 0.195 | 0.346 | 0.646 | 0.049 | 0.039 |
| p-value‡ | 0.424 | 0.425 | 0.322 | 0.172 | 0.044 | 0.042 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download