Abstract
Purpose
To evaluate the 3-year visual and morphological outcomes of diabetic macular edema (DME) based on the morphological pattern observed on optical coherence tomography (OCT) after intravitreal ranibizumab injections.
Methods
Thirty-two eyes of 32 patients with DME were classified according to the following OCT features: diffuse retinal thickening (DRT), cystoid macular edema (CME), and serous retinal detachment (SRD). All patients received 3 consecutive monthly intravitreal injections of 0.5 mg ranibizumab. After 3 injections, patients received ranibizumab or dexamethasone implantation as needed. The primary outcome was the number of treatments received based on the DME type over 36 months. Best-corrected visual acuity (BCVA), central subfoveal thickness, and macular volume changes were also evaluated.
Results
The eyes were classified as DRT (n = 16), CME (6), or SRD (10). The mean number of injections over 3 years was significantly different among the groups: DRT (4.25), CME (7.5), SRD (7.6; p = 0.048). The number of patients who did not need additional treatment after the initial 3 injections was 13 with DRT (81.3%), 2 with CME (33.3%), and 5 with SRD (50%; p = 0.045). BCVA at 36 months significantly improved from baseline in the DRT group (p = 0.003). The CME group showed the worst BCVA among the groups (p = 0.023). Six patients who received intravitreal dexamethasone implantation showed no significant improvement of BCVA but significant decrease in macular volume from 12 to 36 months.
Conclusions
Clinical courses varied according to the morphological pattern of DME after intravitreal ranibizumab injection, and patients with DRT maintained visual and anatomical improvement with fewer injections over 36 months. Additional dexamethasone implantation showed limited effect in reducing macular edema with persistent macular cystic change, but no significant improvement in visual acuity.
References
1. Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and abdominal macular edema: pathophysiology, screening, and novel therapies. Diabetes Care. 2003; 26:2653–64.
2. Nentwich MM, Ulbig MW. Diabetic retinopathy – ocular abdominals of diabetes mellitus. World J Diabetes. 2015; 6:489–99.
3. Tranos PG, Wickremasinghe SS, Stangos NT, et al. Macular edema. Surv Ophthalmol. 2004; 49:470–90.

4. Otani T, Kishi S, Maruyama Y. Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol. 1999; 127:688–93.

5. Scholl S, Augustin A, Loewenstein A, et al. General abdominal of macular edema. Eur J Ophthalmol. 2011; 21(Suppl 6):S10–9.
6. Yanoff M, Fine BS, Brucker AJ, Eagle RC Jr. Pathology of human cystoid macular edema. Surv Ophthalmol. 1984; 28(Suppl):505–11.

7. Lim JH, Kim IH, Bae GH, et al. Analysis of optical coherence tomographic patterns and clinical courses in diabetic macular edema after treatment. J Korean Ophthalmol Soc. 2014; 55:222–9.

8. Kang SW, Park CY, Ham DI. The correlation between fluorescein angiographic and optical coherence tomographic features in abdominally significant diabetic macular edema. Am J Ophthalmol. 2004; 137:313–22.
9. Shimura M, Yasuda K, Yasuda M, Nakazawa T. Visual outcome abdominal intravitreal bevacizumab depends on the optical coherence tomographic patterns of patients with diffuse diabetic macular edema. Retina. 2013; 33:740–7.
10. Wu PC, Lai CH, Chen CL, Kuo CN. Optical coherence abdominal patterns in diabetic macula edema can predict the effects of intravitreal bevacizumab injection as primary treatment. J Ocul Pharmacol Ther. 2012; 28:59–64.
11. Kim M, Lee P, Kim Y, et al. Effect of intravitreal bevacizumab based on optical coherence tomography patterns of diabetic abdominal edema. Ophthalmologica. 2011; 226:138–44.
12. Kim SH, Park JM. Comparison of intravitreal triamcinolone versus bevacizumab in bilateral diabetic macular edema by optical abdominal tomography (OCT) patterns. J Korean Ophthalmol Soc. 2010; 51:210–9.
13. Kim YG, Yu SY, Kwak HW. The effect of intravitreal triamcinolone acetonide injection according to the diabetic macular edema type. J Korean Ophthalmol Soc. 2005; 46:84–9.
14. Yoon SC, Lee DY, Nam DH. The effect of intravitreal abdominal injection according to the OCT patterns of diabetic macular edema. J Korean Ophthalmol Soc. 2008; 49:1611–8.
15. Das A, McGuire PG, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology. 2015; 122:1375–94.

16. Dong N, Xu B, Wang B, Chu L. Study of 27 aqueous humor abdominal in patients with type 2 diabetes with or without retinopathy. Mol Vis. 2013; 19:1734–46.
17. Sohn HJ, Han DH, Kim IT, et al. Changes in aqueous abdominal of various cytokines after intravitreal triamcinolone versus bevacizumab for diabetic macular edema. Am J Ophthalmol. 2011; 152:686–94.
18. Funatsu H, Noma H, Mimura T, et al. Association of vitreous abdominal factors with diabetic macular edema. Ophthalmology. 2009; 116:73–9.
19. Yoshimura T, Sonoda KH, Sugahara M, et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One. 2009; 4:e8158.

20. Sarao V, Veritti D, Boscia F, Lanzetta P. Intravitreal steroids for the treatment of retinal diseases. Scientific World Journal. 2014; 2014:989501.

21. Felinski EA, Cox AE, Phillips BE, Antonetti DA. Glucocorticoids induce transactivation of tight junction genes occludin and clau-din-5 in retinal endothelial cells via a novel cis-element. Exp Eye Res. 2008; 86:867–78.

22. Lee SH, Kim SY, Park HS. abdominal results of dexamethasone intravitreal implant in patients with refractory diabetic macular edema. J Korean Ophthalmol Soc. 2015; 56:1201–7.
23. Lazic R, Lukic M, Boras I, et al. Treatment of anti-vascular endo.
Figure 1.
Morphologic patterns of diabetic macular edema by optical coherence tomography. (A) Diffuse retinal thickening type looks as a sponge-like swelling area with reduced retinal reflectivity. (B) Cystoid macular edema type shows intraretinal cystoid spaces. (C) Serous retinal detachment exhibits elevation of retina and fluid is accumulated between retina and retinal pigment epithelium.
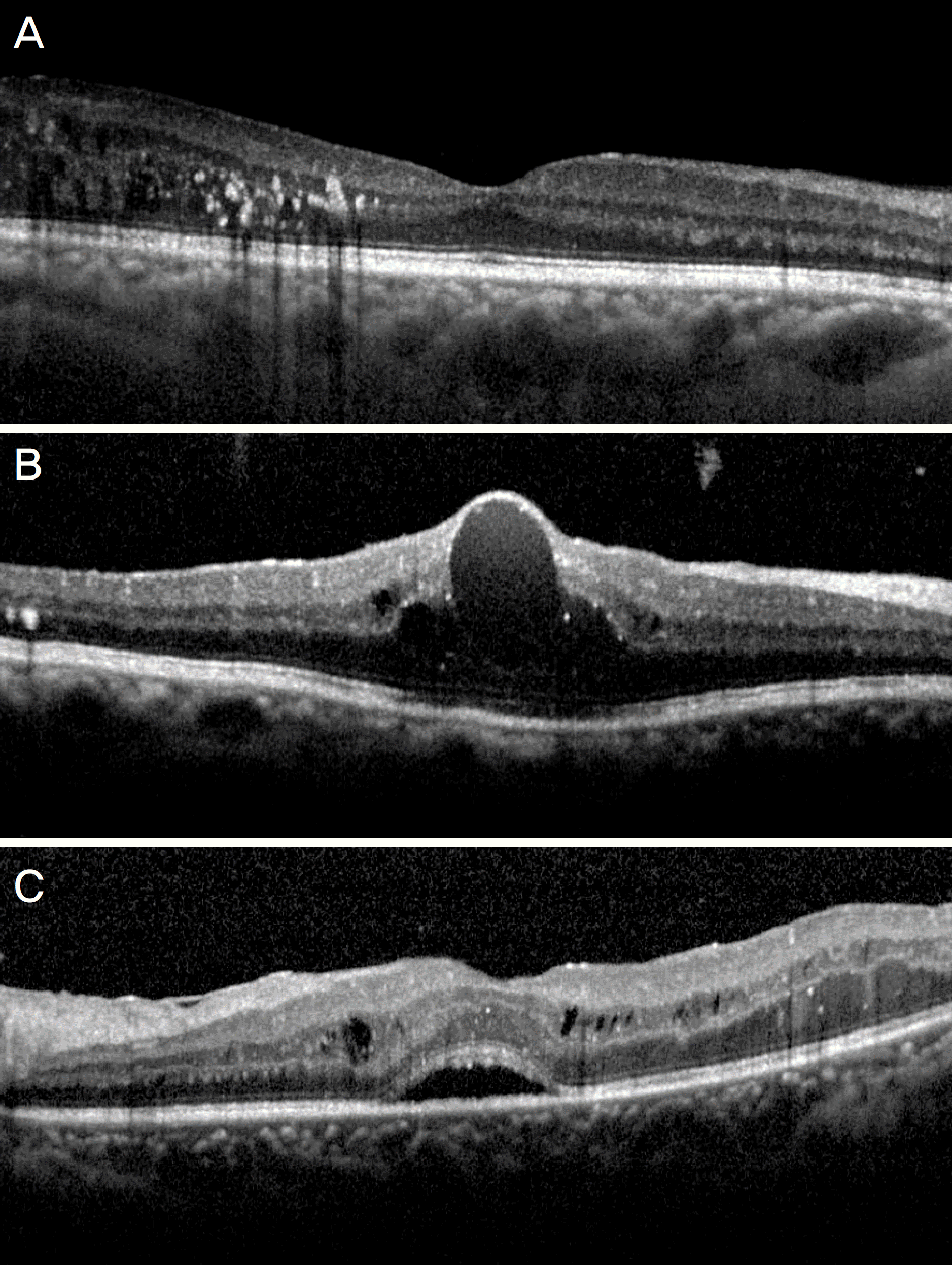
Figure 2.
Change in the mean best-corrected visual acuity (BCVA) over time after intravitreal Ranibizumab injection. In diffuse retinal thickening (DRT) type, significant improvement of BCVA was maintained from 3 to 36 months. In cystoid macular edema (CME) type, BCVA significantly improved over 12 months, but there was no significant difference with baseline from 18 to 36 months. serous retinal detachment (SRD) type showed significant BCVA improvement at 3 and 18months, which were not maintained over 36 months. SRD type showed poorer BCVA at 6, 12 months, and CME type showed poorer BCVA from 18 to 36 months than the other types. Error bar indicates 95% confidence interval.
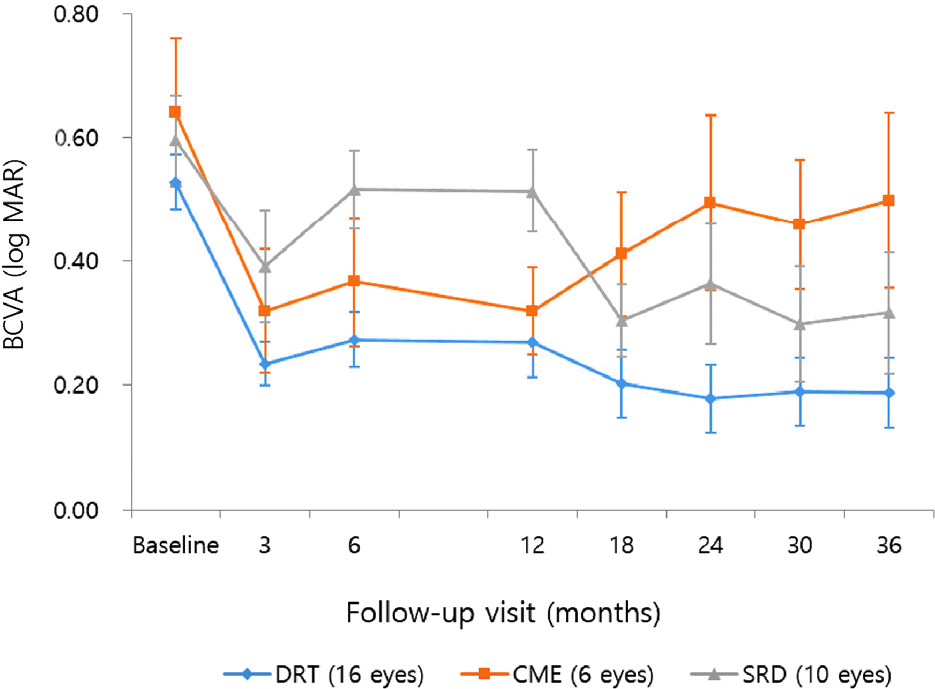
Figure 3.
Change in the mean central subfoveal thickness (CST) over time after intravitreal Ranibizumab injection. In diffuse retinal thickening (DRT) and serous retinal detachment (SRD) types, CST significantly improved from the baseline through 36 months, except 18 months of SRD type. In cystoid macular edema (CME) type, significant improvement of CST was not maintained after 6 months. There was no difference in the CST between the individual types. Error bar indicates 95% confidence interval.
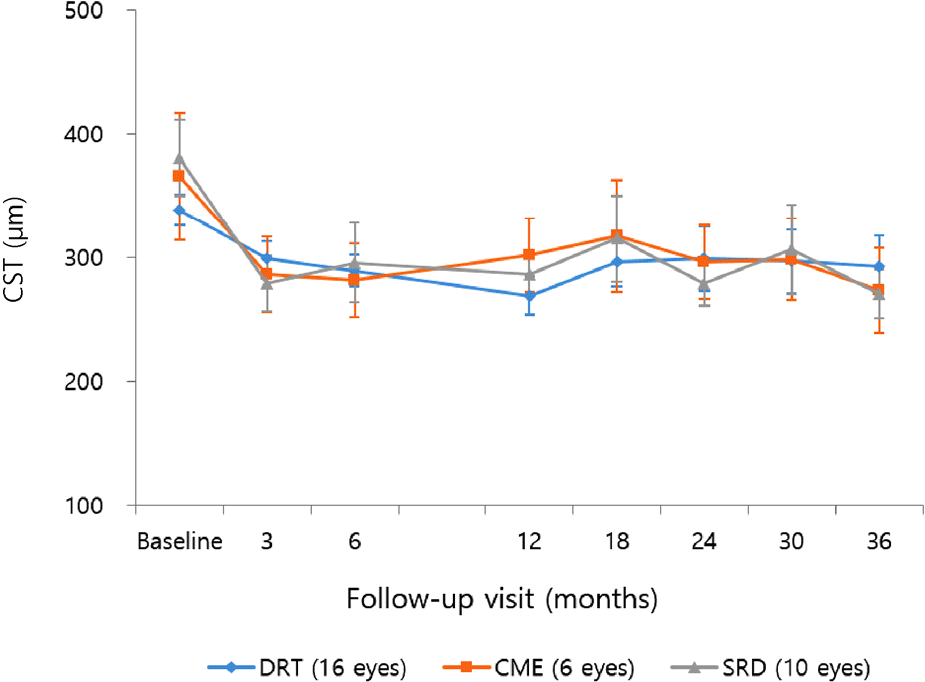
Figure 4.
Change in the mean macular volume over time after intravitreal Ranibizumab injection. In diffuse retinal thickening (DRT) type, macular volume significantly improved from the baseline until 18 months. In cystoid macular edema (CME) and serous retinal detachment (SRD) types, macular volume significantly deceased at 3 months after treatment, then aggravated until 18 months. However both types showed significant reduction of macular volume at 36 months compared to baseline. Error bar indicates 95% confidence interval.
Figure 5.
Change in the mean best-corrected visual acuity (BCVA), central subfoveal thickness (CST), and macular volume over time in Dexamethasone implantation added patients. (A) Mean BCVA was aggravated at 36 months (0.6) compared to 12 months, which was not significant (0.48) (p = 0.322). (B) Mean CST did not showed significant difference between 12 and 36 months (p = 0.833). (C) Mean macular volume significantly decreased from 12 months (12.1 mm3) to 36 months (11.38 mm3) (p = 0.001). Error bar indicates 95% confidence interval.
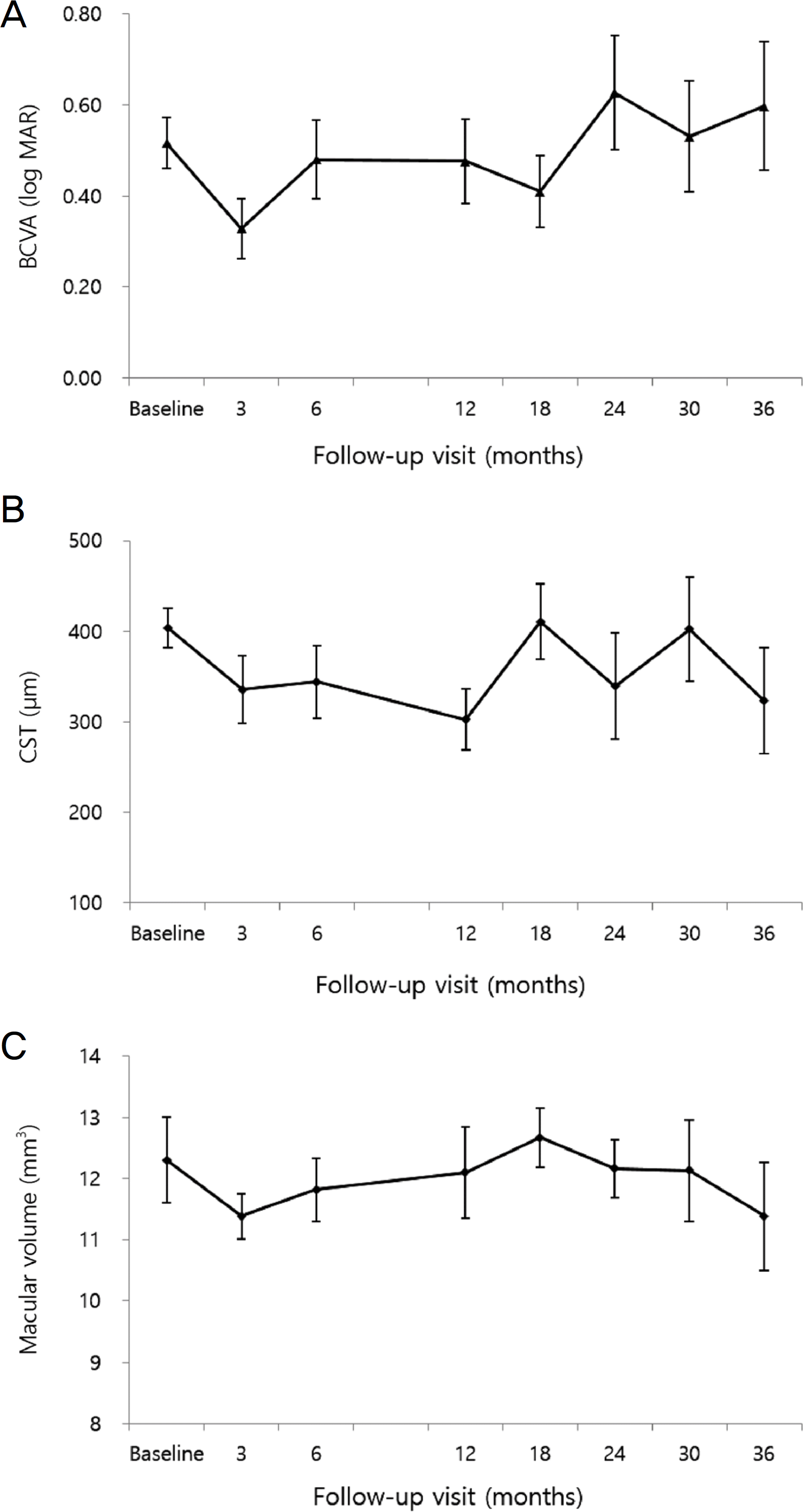
Table 1.
Baseline characteristics of eyes and optical coherence tomography findings
| DRT | CME | SRD | p-value | |
|---|---|---|---|---|
| Eyes (n, %) | 16 (50) | 6 (18.8) | 10 (31.2) | |
| Mean Age (years) | 59.81 ±11.02 | 61.17 ± 8.38 | 61.30 ± 10.94 | 0.979* |
| Mean HbA1C (%) | 7.86 ± 1.59 | 8.10 ± 1.66 | 8.67 ± 1.60 | 0.353* |
| Diabetic retinopathy severity (n, %) | ||||
| PDR | 5 (31.3) | 2 (33.3) | 3 (30) | 1.000† |
| NPDR | 11 (68.7) | 4 (66.7) | 7 (70) | |
| Insulin treatment (n, %) | 7 (43.8) | 2 (33.3) | 7 (70) | 0.370† |
| Mean time since diagnosis of diabetes (years) | 15.00 ± 6.90 | 12.17 ± 4.62 | 13.40 ± 5.21 | 0.581* |
| Mean time since diagnosis of DME (years) | 1.30 ± 1.11 | 1.92 ± 1.85 | 1.91 ± 1.08 | 0.431* |
| Mean BCVA (log MAR) | 0.52 ± 0.16 | 0.64 ± 0.29 | 0.60 ± 0.23 | 0.062* |
| Mean CST (μ m) | 338.44 ± 49.20 | 365.67 ± 126.2 | 380.6 ± 96.81 | 0.277* |
| Mean macular volume (mm3) | 10.84 ± 0.90 | 11.72 ± 1.15 | 12.43 ± 2.09 | 0.058* |
DRT = diffuse retinal thickening; CME = cystoid macular edema; SRD = serous retinal detachment; PDR = proliferative diabetic retinopathy; NPDR = nonproliferative diabetic retinopathy; DME = diabetic macular edema; BCVA = best-corrected visual acuity; log MAR = logarithm of the minimum angle of resolution; CST = central subfoveal thickness.
Table 2.
Mean number of intravitreal treatments received over 3 years
| DRT | CME | SRD | p-value | |
|---|---|---|---|---|
| Mean number of Ranibizumab treatments (n) | 4.68 ± 0.95 | 7.50 ± 2.88 | 7.60 ± 3.98 | 0.048* |
| (Day 1– Month 36) | ||||
| Mean number of Ranibizumab treatments (n) | 3.50 ± 0.73 | 5.16 ± 1.94 | 4.90 ± 2.28 | 0.074* |
| (Day 1– Month 12) | ||||
| Mean number of Ranibizumab treatments (n) | 1.19 ± 0.73 | 2.33 ± 0.49 | 2.70 ± 0.68 | 0.037* |
| (Month 12– Month 36) | ||||
| Eyes, no additional injections (n) | 13 (81.3%) | 2 (33.3%) | 5 (50%) | 0.045† |
| (Month 12– Month 36) | ||||
| Eyes, received Dexamethasone implantation (n) | 1 (5.95%) | 2 (33.3%) | 3 (30%) | 0.222† |
| (Month 12– Month 36) | ||||
| Mean number of Dexamethasone implantation (n) | 1 | 1.5 | 1.67 | |
| (Month 12– Month 36) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download