Abstract
Purpose
To evaluate the effect of intravitreal aflibercept according to subfoveal choroidal thickness in patients with polypoidal choroidal vasculopathy (PCV).
Methods
We retrospectively analyzed the medical records of 60 eyes from 60 patients with PCV treated with intravitreal aflibercept. The patients were followed for at least 6 months after the first injection. Using software, subfoveal choroidal thick-ness was manually measured as the distance from the hyperreflective line of Bruch's membrane to the chorioscleral interface on optical coherence tomography. The patients were divided into three groups based on subfoveal choroidal thickness. Visual acuity, subfoveal choroidal thickness, central macular thickness and largest pigment epithelial detachment (PED) height, polyp regression rate, and dry macula rate were evaluated to analyze the anatomical and functional outcomes.
Results
Baseline mean subfoveal choroidal thickness were 178.50 ± 28.42 μ m in the thin group (14 eyes, 23.3%), 287.03 ±43.58 μ m in the medium group (33 eyes, 55.0%), and 379.77 ± 17.09 μ m in the thick group (13 eyes, 21.7%). Baseline age, sex, visual acuity, central macular thickness, and the largest PED height did not differ significantly among the three subgroups. Only the thin group showed significant improvement of visual acuity at 6 months (p = 0.005). Subfoveal choroidal thickness, central macular thickness, and largest PED height were significantly decreased after treatment in all subgroups and did not differ among the subgroups. Compared with the other groups, the thin subfoveal choroidal thickness group showed higher polyp regression rate at 3 months and higher dry macula rate at 6 months (p = 0.013 and p = 0.004, respectively).
Go to : 
References
1. Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic abdominal choroidal vasculopathy (IPCV). Retina. 1990; 10:1–8.
2. Ciardella AP, Donsoff IM, Huang SJ, et al. Polypoidal choroidal vasculopathy. Surv Ophthalmol. 2004; 49:25–37.

3. Lee JW, Kim IT. Epidemiologic and clinical characteristics of abdominal choroidal vasculopathy in Korean patients. J Korean Ophthalmol Soc. 2007; 48:63–74.
4. Wong CW, Yanagi Y, Lee WK, et al. Age-related macular abdominal and polypoidal choroidal vasculopathy in Asians. Prog Retin Eye Res. 2016; 53:107–39.
5. Koh A, Lee WK, Chen LJ, et al. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012; 32:1453–64.
6. Yamamoto A, Okada AA, Kano M, et al. One-year results of abdominal aflibercept for polypoidal choroidal vasculopathy. Ophthalmology. 2015; 122:1866–72.
7. Oishi A, Tsujikawa A, Yamashiro K, et al. One-year result of abdominal treatment on age-related macular degeneration and abdominal factors for visual outcome. Am J Ophthalmol. 2015; 159:853–60.e1.
8. Introini U, Casalino G, Triolo G, et al. Stereotactic radiotherapy for polypoidal choroidal vasculopathy: a pilot study. Ophthalmologica. 2015; 233:82–8.

9. Inoue M, Yamane S, Taoka R, et al. Aflibercept for polypoidal abdominal vasculopathy: as needed versus fixed interval dosing. Retina. 2016; 36:1527–34.
10. Sasahara M, Tsujikawa A, Musashi K, et al. Polypoidal choroidal vasculopathy with choroidal vascular hyperpermeability. Am J Ophthalmol. 2006; 142:601–7.

11. Jirarattanasopa P, Ooto S, Nakata I, et al. Choroidal thickness, abdominal hyperpermeability, and complement factor H in age-related macular degeneration and polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2012; 53:3663–72.
12. Yuzawa M, Mori R, Kawamura A. The origins of polypoidal abdominal vasculopathy. Br J Ophthalmol. 2005; 89:602–7.
13. Koizumi H, Yamagishi T, Yamazaki T, Kinoshita S. Relationship between clinical characteristics of polypoidal choroidal abdominal and choroidal vascular hyperpermeability. Am J Ophthalmol. 2013; 155:305–13.e1.
14. Gallego-Pinazo R, Dolz-Marco R, Gómez-Ulla F, et al. Pachychoroid diseases of the macula. Med Hypothesis Discov Innov Ophthalmol. 2014; 3:111–5.
15. Kuroda S, Ikuno Y, Yasuno Y, et al. Choroidal thickness in central serous chorioretinopathy. Retina. 2013; 33:302–8.

18. Yang LH, Jonas JB, Wei WB. Optical coherence tomographic abdominal depth imaging of polypoidal choroidal vasculopathy. Retina. 2013; 33:1584–9.
19. Balaratnasingam C, Lee WK, Koizumi H, et al. Polypoidal abdominal vasculopathy: a distinct disease or manifestation of many? Retina. 2016; 36:1–8.
20. Chung SE, Kang SW, Lee JH, Kim YT. Choroidal thickness in abdominal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 2011; 118:840–5.
21. Shin JY, Kwon KY, Byeon SH. Association between choroidal thickness and the response to intravitreal ranibizumab injection in age‐ related macular degeneration. Acta Ophthalmol. 2015; 93:524–32.
22. Kang HM, Kwon HJ, Yi JH, et al. Subfoveal choroidal thickness as a potential predictor of visual outcome and treatment response after intravitreal ranibizumab injections for typical exudative age-abdominal macular degeneration. Am J Ophthalmol. 2014; 157:1013–21.
23. Saito M, Kano M, Itagaki K, et al. Switching to intravitreal aflibercept injection for polypoidal choroidal vasculopathy refractory to ranibizumab. Retina. 2014; 34:2192–201.

24. Yang H, Jeon HM, Kim SW, et al. abdominal efficacy of abdominal aflibercept for polypoidal choroidal vasculopathy. J Korean Ophthalmol Soc. 2015; 56:1728–35.
25. Inoue M, Arakawa A, Yamane S, Kadonosono K. abdominal abdominal of intravitreal aflibercept in treatment-naive patients with abdominal choroidal vasculopathy. Retina. 2014; 34:2178–84.
26. Lee KH, Lee SC, Lee CS. Reproducibility of choroidal thickness in normal Korean eyes using two spectral domain optical coherence tomography. J Korean Ophthalmol Soc. 2013; 54:1365–70.

27. Kim SW, Oh J, Kwon SS, et al. Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous abdominal, and polypoidal choroidal vasculopathy. Retina. 2011; 31:1904–11.
28. Yuzawa M. Polypoidal choroidal vasculopathy. Nippon Ganka Gakkai Zasshi. 2012; 116:200–31. discussion 232.

29. Tanaka K, Nakayama T, Mori R, et al. Associations of complement factor H (CFH) and age-related maculopathy susceptibility 2 (ARMS2) genotypes with subtypes of polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2011; 52:7441–4.

30. Kawamura A, Yuzawa M, Mori R, et al. Indocyanine green abdominal and optical coherence tomographic findings support abdominal of polypoidal choroidal vasculopathy into two types. Acta Ophthalmol. 2013; 91:e474–81.
31. Coscas G, Lupidi M, Coscas F, et al. Toward a specific abdominal of polypoidal choroidal vasculopathy: idiopathic disease or subtype of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015; 56:3187–95.
Go to : 
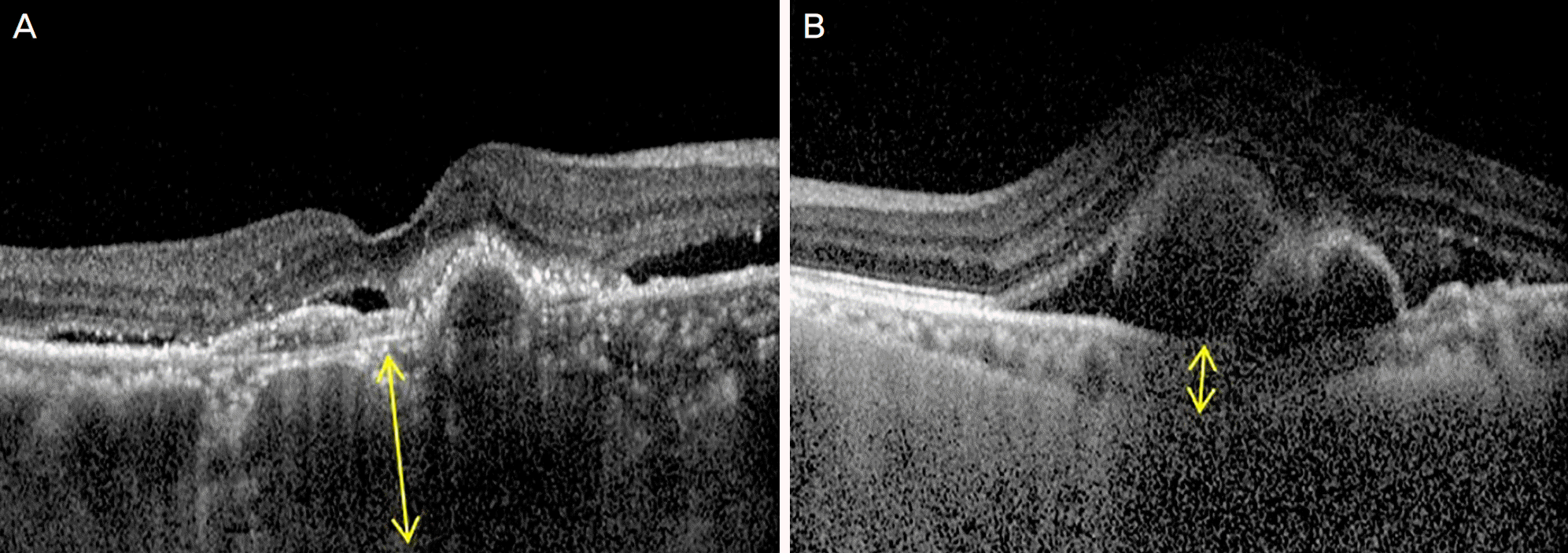 | Figure 1.Choroidal thickness was measured manually using a caliper that is provided in the optical coherence tomography device. The choroidal thickness (yellow arrow) was defined as the vertical distance between Bruch's membrane and the chorioscleral interface. (A) Thick choroidal thickness. (B) Thin choroidal thickness. |
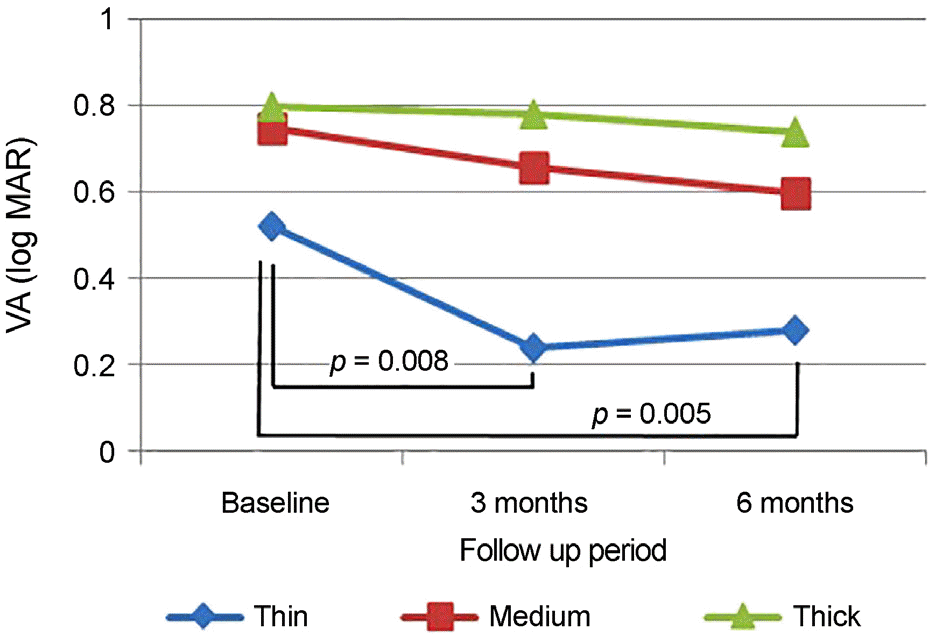 | Figure 2.Subtype comparison of changes in best corrected visual acuity (log MAR) after intravitreal aflibercept injection. Thin group patients had statistically significant improvement of best corrected visual acuity at 3 months and 6 months compared to the baseline (3 months p = 0.008, 6 months p = 0.005, paired t-test). There was no statistically significant improvement in medium group and thick group. VA = visual acuity. |
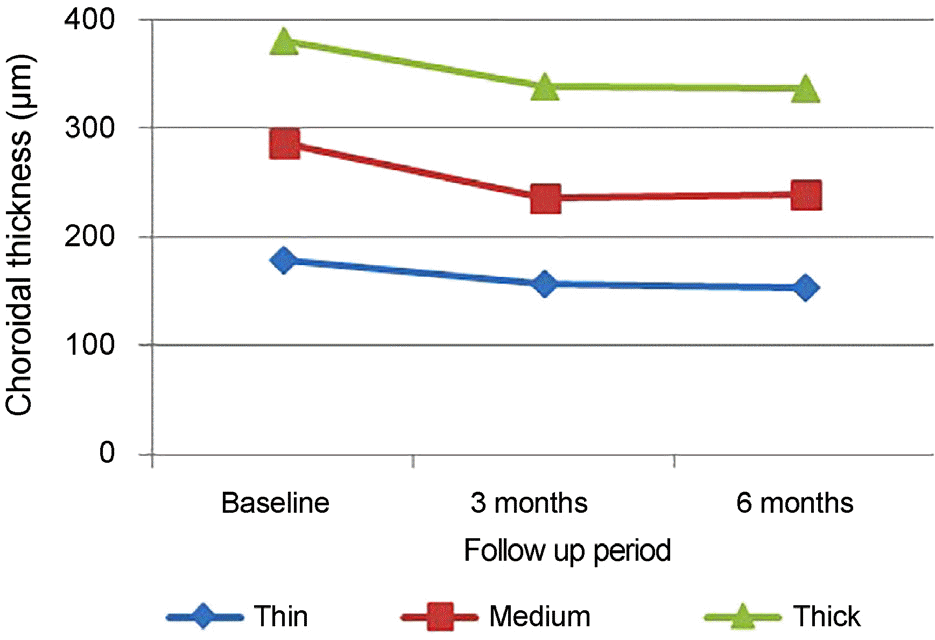 | Figure 3.Subtype comparison of changes in subfoveal choroidal thickness (μ m) after intravitreal aflibercept injection. Choroidal thickness of all each group significantly decreased at 3 months and 6 months compared to the baseline. No stat-istical differences were shown among 3 groups for 6 months. |
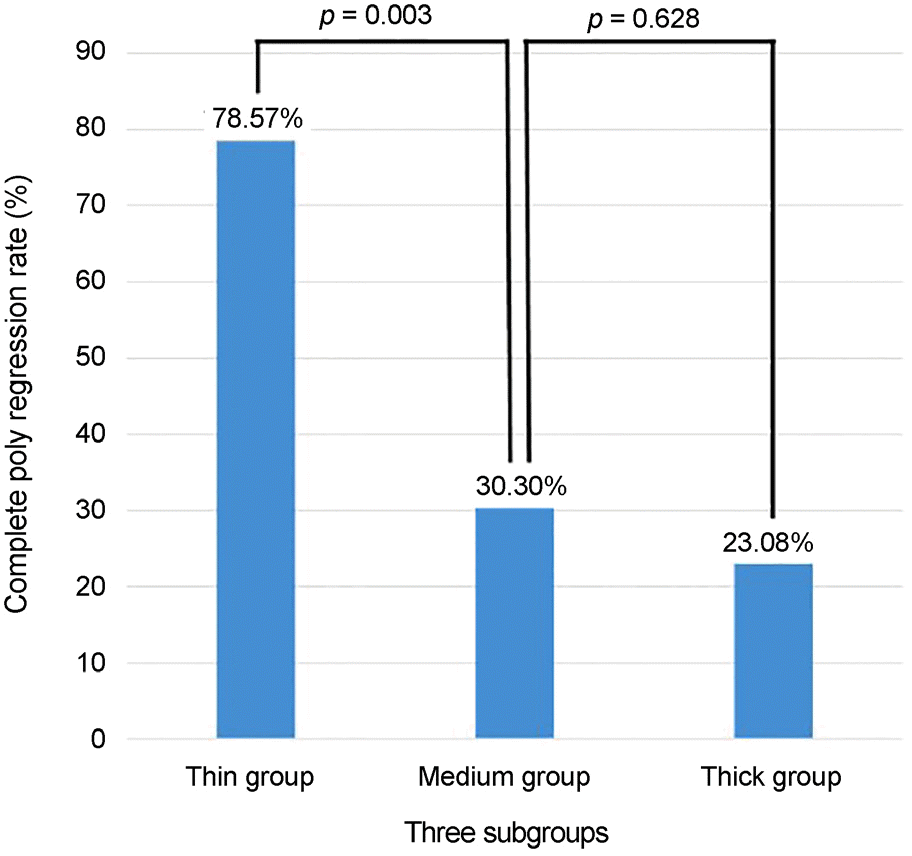 | Figure 4.Subtype comparison of complete polyp regression rate at 3 months after 1st intravitreal aflibercept injection. The thin subfoveal choroidal thickness group showed higher complete polyp regression rate at 3 months. |
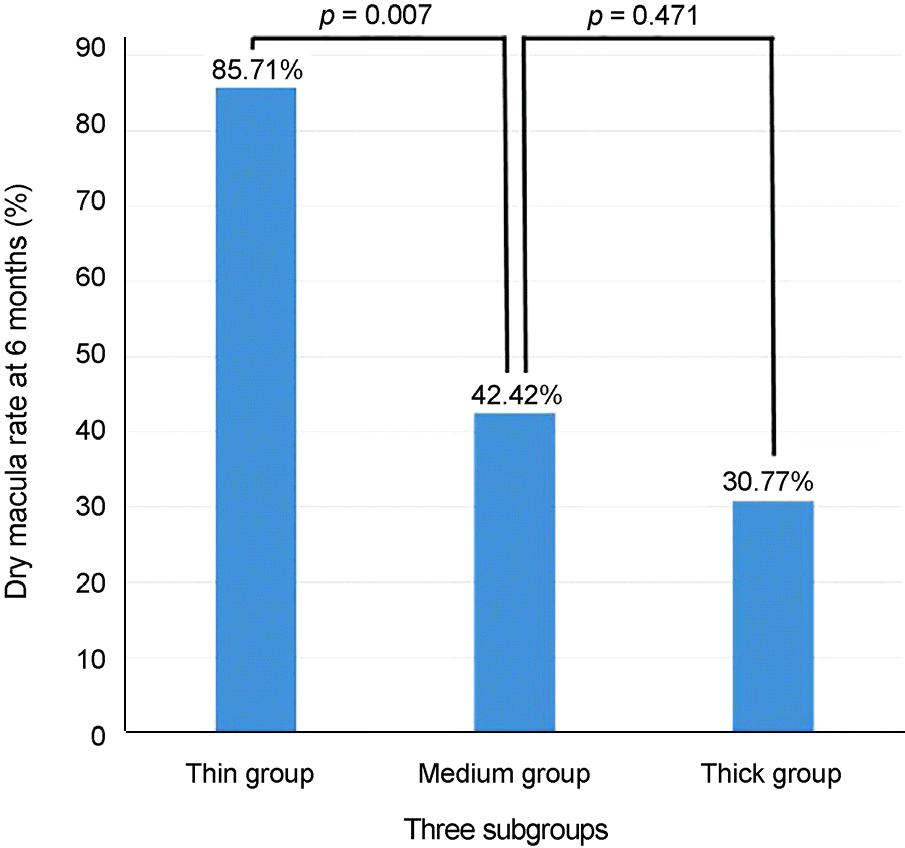 | Figure 5.Subtype comparison of anatomical outcomes using dry macula rate at 6 months after 1st intravitreal aflibercept injection. The thin subfoveal choroidal thickness group showed higher dry macula rate at 6 months. |
Table 1.
Baseline characteristics
| Total | Thin group | Medium group | Thick group | p-value | |
|---|---|---|---|---|---|
| Number of patients Mean F/U period (months) | 60 10.8 ± 4.96 | 14 (23.3%) 9.50 ± 4.94 | 33 (55.0%) 10.4 ± 4.74 | 13 (21.7%) 13.2 ± 5.13 | 0.130† |
| Age (years) | 68.63 ± 7.42 | 71.21 ± 7.66 | 68.03 ± 7.37 | 67.38 ± 7.21 | 0.325† |
| Gender (male:female) | 51 (85%):9 (15%) | 13:1 (92.9%:7.1%) | 28:5 (84.8%:15.2%) | 10:3 (76.9%:23.1%) | 0.489* |
| Number of injections for 6 months | |||||
| Average number of injection | 3.71 ± 0.47 | 3.82 ± 0.39 | 3.92 ± 0.28 | 0.387† | |
| Number of eyes 3 times injected | 4 (28.6) | 6 (18.2) | 1 (7.7) | 0.058‡ | |
| Number of eyes 4 times injected | 10 (71.4) | 27 (81.8) | 12 (92.3) | 0.058‡ | |
| Refractive errors (SE, diopters) | 0.56 ± 0.99 | 0.59 ± 0.81 | 0.46 ± 1.11 | 0.77 ± 0.86 | 0.620† |
| Baseline VA (log MAR) | 0.71 ± 0.43 | 0.52 ± 0.37 | 0.75 ± 0.46 | 0.80 ± 0.36 | 0.124† |
| Baseline subfoveal choroidal thickness (μ m) | 281.80 ± 77.00 | 178.50 ± 28.42 | 287.03 ± 43.58 | 379.77 ± 17.09 | <0.001† |
| Baseline central macular thickness (μ m) | 456.87 ± 129.39 | 407.29 ± 100.45 | 464.03 ± 139.45 | 492.08 ± 123.18 | 0.136† |
| Baseline largest PED height (μ m) | 317.02 ± 174.86 | 292.29 ± 145.03 | 289.09 ± 186.36 | 414.54 ± 147.56 | 0.052† |
Table 2.
Changes of visual acuity, subfoveal thickness, central macular thickness and largest PED height of 60 eyes after intravitreal aflibercept injection
| Baseline | 3 months | 6 months | |
|---|---|---|---|
| VA (log MAR) | 0.71 ± 0.43 | 0.59 ± 0.44 | 0.56 ± 0.40 |
| p-value | 0.007* | <0.001* | |
| Subfoveal choroidal thickness (μ m) | 281.80 ± 77.00 | 239.12 ± 77.66 | 240.20 ± 77.69 |
| p-value | <0.001* | <0.001* | |
| Central macular thickness (μ m) | 456.87 ± 129.39 | 288.33 ± 104.92 | 314.97 ± 97.50 |
| p-value | 0.001† | 0.001† | |
| Largest PED height (μ m) | 317.02 ± 174.86 | 189.50 ± 134.56 | 187.90 ± 131.71 |
| p-value | <0.001* | <0.001* |
Table 3.
Comparison of baseline indocyanine green angiography findings among 3 subgroups
| Thin | Medium | Thick | p-value | |
|---|---|---|---|---|
| Location of polyps | 0.499* | |||
| Subfovea | 4 (28.6) | 11 (33.3) | 3 (23.1) | |
| Parafovea | 9 (64.3) | 15 (45.5) | 5 (38.5) | |
| Perifovea | 1 (7.1) | 6 (18.2) | 3 (23.1) | |
| Outside from perifovea | 0 (0) | 1 (3.0) | 2 (15.4) | |
| Number of polyps | 3.79 ± 3.26 | 3.15 ± 2.65 | 3.69 ± 1.70 | 0.401† |
| Size of polyps (μ m) | 150.77 ± 54.62 | 172.61 ± 73.31 | 215.00 ± 70.90 | 0.305† |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download