Abstract
Purpose
To investigate the effects of intranasal steroid spray after silicone tube intubation in nasolacrimal duct obstruction.
Methods
We included 73 patients (73 eyes) who had undergone silicone tube intubation with partial nasolacrimal duct obstruction and who had been followed-up for more than 6 months. We divided them into two groups: Group 1 (37 patients, 37 eyes), who used intranasal steroid spray twice a day for 4 weeks after silicone tube intubation, and Group 2 (36 patients, 36 eyes), who did not use intranasal steroid spray. A retrospective medical record review was performed to analyze the clinical features of epiphora improvement and complications in the two groups.
Results
No significant difference was found in epiphora improvement after surgery between the two groups (33 eyes [89.2%] in Group 1, 31 eyes [86.1%] in Group 2) (p = 0.736). In addition, the difference in success rate between the two groups was also not statistically significant (33 eyes [89.2%] in Group 1, 28 eyes [77.8%] in Group 2) (p = 0.221). Group 1 (5/37 [13.5%] eyes) and Group 2 (12/36 [33.3%] eyes) complained of ocular discomfort during the period of silicone tube intubation (p = 0.045). The complication rate of Group 1 (5/37 [13.5%] eyes) was significantly lower than that of Group 2 (13/36 [36.1%] eyes) (p = 0.024).
Conclusions
Silicone tube intubation is an effective treatment option for adults diagnosed with partial nasolacrimal duct obstruction, and postoperative intranasal steroid application may contribute to improvement of ocular symptoms after the surgery. There may be a merit of using intranasal steroid spray for adjuvant therapy to prevent postoperative complications, but it needs further study considering various factors.
Go to : 
References
1. al-Hussain H, Nasr AM. Silastic Intubation in congenital abdominal duct obstruction: a study of 129 eyes. Ophthal Plast Reconstr Surg. 1993; 9:32–7.
2. Pashby RC, Rathbun JE. Silicone tube intubation of the lacrimal drainage system. Arch Ophthalmol. 1979; 97:1318–22.

3. McDonogh M, Meiring JH. Endoscopic transnasal dacryocystorhinostomy. J Laryngol Otol. 1989; 103:585–7.

4. Pack YM, Koo GH, Lee JE, et al. Comparision of clinical efficacy between tie methods of silicone tube intubation in nasolacrimal duct obstruction. J Korean Ophthalmol Soc. 2009; 50:177–81.
5. Lee JH, Kang MS, Yang JW. Clinicopathologic findings after abdominal polyurethane stent implantations. Korean J Ophthalmol. 2005; 19:252–7.
6. Holmberg K, Karlsson G. Nasal polyps: medical or surgical abdominal? Clin Exp Allergy. 1996; 26(Suppl 3):23–30.
7. Ingber DE, Madri JA, Folkman J. A possible mechanism for abdominal of angiogenesis by angiostatic steroids: induction of abdominal basement membrane dissolution. Endocrinology. 1986; 119:1768–75.
8. Gagne D, Pons M, Philibert D. RU 38486: a potent anti-glucocorticoid in vitro and in vivo. J Steroid Biochem. 1985; 23:247–51.

9. Anolik R, Nathan RA, Schenkel E, et al. Intranasal mometasone abdominal alleviates the ocular symptoms associated with seasonal abdominal rhinitis: results of a post hoc analysis. Int Arch Allergy Immunol. 2008; 147:323–30.
10. Bielory L. Ocular symptom reduction in patients with seasonal abdominal rhinitis treated with the intranasal corticosteroid abdominal furoate. Ann Allergy Asthma Immunol. 2008; 100:272–9.
11. Rodrigo GJ, Neffen H. Efficacy of fluticasone furoate nasal spray vs. placebo for the treatment of ocular and nasal symptoms of abdominal rhinitis: a systematic review. Clin Exp Allergy. 2011; 41:160–70.
12. Keith PK, Scadding GK. Are intranasal corticosteroids all equally consistent in managing ocular symptoms of seasonal allergic rhini-tis? Curr Med Res Opin. 2009; 25:2021–41.

13. Soll DB. Silicone intubation: an alternative to dacryocystorhinostomy. Ophthalmology. 1978; 85:1259–66.

14. Anderson RL, Edwards JJ. Indications, complications and results with silicone stents. Ophthalmology. 1979; 86:1474–87.

15. Kim DM, Roh KK. Results with silicone stent in lacrimal drainage system. J Korean Ophthalmol Soc. 1987; 28:733–5.
16. Jung JJ, Jang SY, Jang JW, In JH. Comparison results of silicone tube intubation according to syringing and dacryocystography. J Korean Ophthalmol Soc. 2014; 55:1584–8.

17. Cho EH, Park SY, Kook KH. The influence of a silicone tube on tear drainage in patients with healed rhinostomy after dacryocystorhinostomy. J Korean Ophthalmol Soc. 2012; 53:1541–8.

18. Picó G. A modified technique of external dacryocystorhinostomy. Am J Ophthalmol. 1971; 72:679–90.

19. Jeong JG, Ann M. The effect of Mitomycin C instillation after abdominal intubation in adult partial nasolacrimal duct obstruction. J Korean Ophthalmol Soc. 2012; 53:1231–5.
20. Sung HK, Kim JH, Jeong JH. The effect of postoperative intranasal steroid in endonasal dacryocystorhinostomy. J Korean Ophthalmol Soc. 2004; 45:1233–8.
21. Kim DS, Lee YJ. Efficacy of silicone nasolacrimal intubation with mitomycin C treatmentfor treatment of incomplete nasolacrimal duct obstruction. J Korean Ophthalmol Soc. 2006; 47:181–5.
22. Liu D, Bosley TM. Silicone nasolacrimal intubation with mitomycin-C: a prospective randomized, double-masked study. Ophthalmology. 2003; 110:306–10.

23. Apuhan T, Yı ldı rı m YS, Eroglu F, Sipahier A. Effect of mitomycin C on endoscopic dacryocystorhinostomy. J Craniofac Surg. 2011; 22:2057–9.

24. Tsirbas A, Davis G, Wormald PJ. Mechanical endonasal abdominal versus external dacryocystorhinostomy. Ophthal Plast Reconstr Surg. 2004; 20:50–6.
25. Holmberg K, Juliusson S, Balder B, et al. Fluticasone propionate aqueous nasal spray in the treatment of nasal polyposis. Ann Allergy Asthma Immunol. 1997; 78:270–6.

26. Park CH, Na SK, Lim HJ, Jung YG. The effects of steroid and abdominal receptor blocker in initial polyp formation. Korean J Otolaryngol-Head Neck Surg. 2000; 43:1312–7.
27. Keith P, Nieminen J, Hollingworth K, Dolovich J. Efficacy and abdominal of fluticasone propionate nasal drops 400 microgram once daily compared with placebo for the treatment of bilateral polyposis in adults. Clin Exp Allergy. 2000; 30:1460–8.
28. Blaiss MS. Evolving paradigm in the management of allergic rhini-tis-associated ocular symptoms: role of intranasal corticosteroids. Curr Med Res Opin. 2008; 24:821–36.

29. Derendorf H, Nave R, Drollmann A, et al. Relevance of abdominal and pharmacodynamics of inhaled corticosteroids to asthma. Eur Respir J. 2006; 28:1042–50.
30. Sato H, Nave R, Nonaka T, et al. In vitro metabolism of ciclesonide in human nasal epithelial cells. Biopharm Drug Dispos. 2007; 28:43–50.

31. Neffen H, Wingertzahn MA. Ciclesonide, a hypotonic intranasal corticosteroid. Allergy Asthma Proc. 2010; 31(Suppl 1):S29–37.

32. Rohatagi S, Arya V, Zech K, et al. Population pharmacokinetics and pharmacodynamics of ciclesonide. J Clin Pharmacol. 2003; 43:365–78.

33. Bernstein DI, Levy AL, Hampel FC, et al. Treatment with abdominal fluticasone propionate significantly improves ocular abdominals in patients with seasonal allergic rhinitis. Clin Exp Allergy. 2004; 34:952–7.
34. Baroody FM, Foster K, Markarian A, Naclerio R. Nasal ocular reflexes occur after nasal challenge with allergen. J Allergy Clin Immunol. 2007; 119:S162.

35. Ratner PH, Wingerzahn MA, van Bavel JH, et al. Efficacy and safety of ciclesonide nasal spray for the treatment of seasonal abdominal rhinitis. J Allergy Clin Immunol. 2006; 118:1142–8.
Go to : 
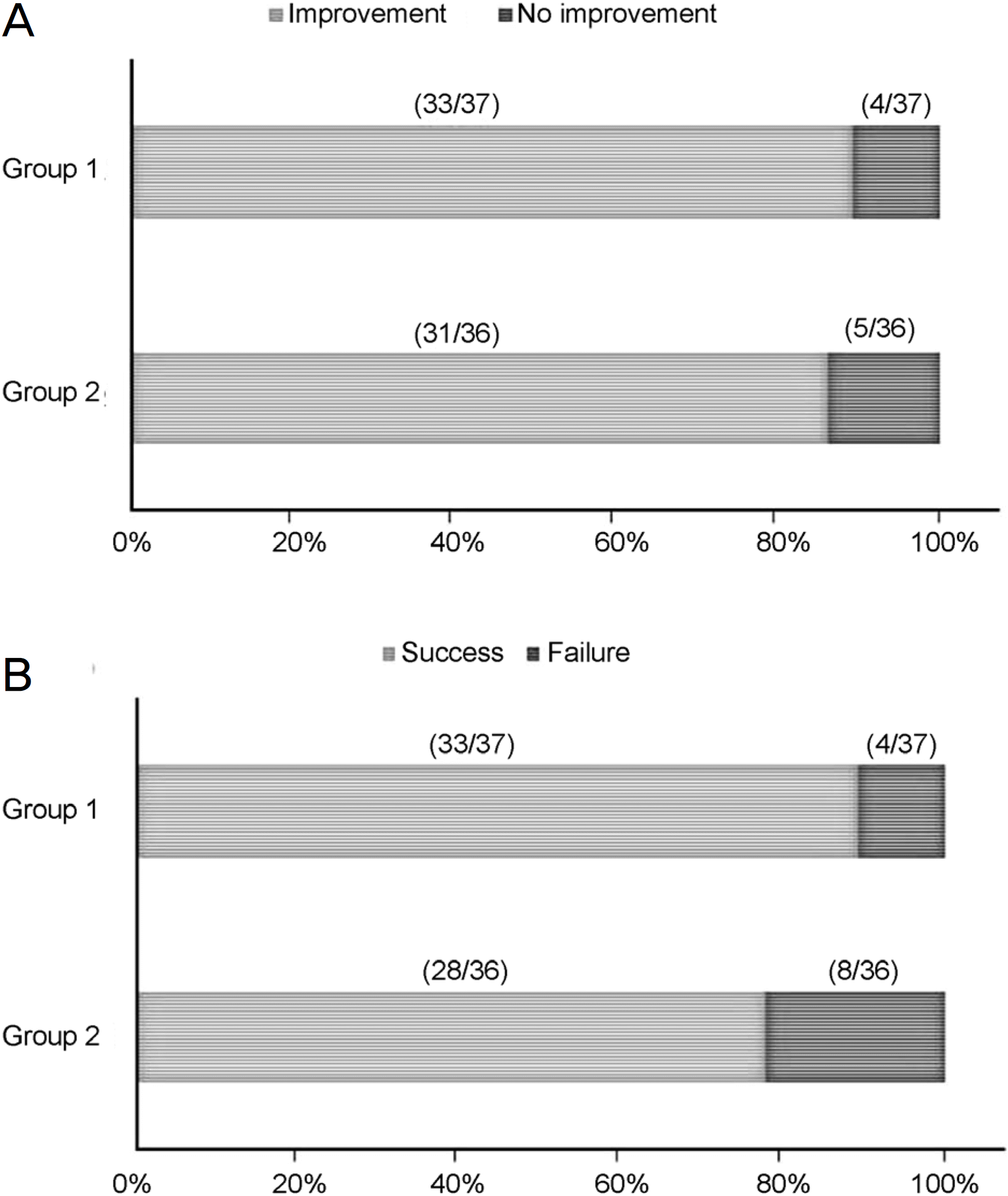 | Figure 1.The rate of epiphora improvement and clinical success outcome in patients used cicleosonide after silicone tube intubation. (A) Epiphora improvement rate, (B) Clinical success outcome rate. But there was no significant difference between group 1 and group 2 (p = 0.736 [A], p = 0.221 [B], Fisher's exact test). |
Table 1.
Baseline characteristics of nasolacrimal duct obstruction patients
| Baseline characteristic | Group 1 (silicone tube intubation + intranasal spray) | Group 2 (silicone tube intubation) | p-value |
|---|---|---|---|
| Total No. of patients | 37 | 36 | |
| Sex (M/F) | 6/31 | 12/24 | 0.109* |
| Age (years) | 63.54 ± 10.62 | 62.25 ± 8.53 | 0.570† |
| Mean BCVA at diagnosis (log MAR) | 0.15 ± 0.14 | 0.14 ± 0.12 | 0.969† |
| Hypertension (n, %) | 11 (29.7) | 8 (22.2) | 0.322* |
| Diabetic mellitus (n, %) | 5 (13.5) | 5 (13.9) | 0.614* |
| Duration of symptom (months) | 14.4 ± 22.7 | 12.9 ± 24.8 | 0.686† |
| Duration of intubation (months) | 5.52 ± 0.81 | 5.14 ± 0.72 | 0.786† |
Table 2.
Comparative complications after silicone tube intubation between two groups
| Group 1 (Silicone tube intubation + Intranasal spray) | Group 2 (Silicone tube intubatio | n) p-value* | |
|---|---|---|---|
| Complication (n, %) | |||
| Mucoplurent discharge | 1 (2.7) | 4 (11.1) | 0.199 |
| Granuloma | 0 (0.0) | 3 (8.4) | 0.115 |
| Conjunctivitis | 2 (5.4) | 3 (8.4) | 0.674 |
| Corneal erosion | 1 (2.7) | 1 (2.8) | 1.000 |
| Canaliculitis | 1 (2.7) | 2 (5.6) | 0.615 |
| Total | 5 (13.5) | 13 (36.1) | 0.024 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download