Abstract
Purpose:
To evaluate the neuroprotective effects of betaxolol (betaxolol hydrochloride) under hypoxic conditions using retinal ganglion cells (RGC-5) and determine whether heme oxygenase-1 (HO-1) expression exerts cytoprotective effects.
Methods
In this study, cultured RGC-5 cells were incubated with different concentrations of betaxolol hydrochloride (0.1 μ M, 1 μ M or 5 μ M) and with 10 μ M zinc protoporphyrin (ZnPP), in a hypoxia incubator (1% O2, 5% CO2, 94% N2) for 48 hours and the cell viability of each group was determined. Additionally, cell viability was measured after RGC-5 cells were incubated with 5 μ M of brinzolamide (Azopt®), brimonidine tartrate (Alphagan®) or travoprost (Travatan®). RGC-5 cells were divided into three groups and incubated under three different conditions, normoxia group (20% O2, 5% CO2), hypoxia group (1% O2, 5% CO2) and the group with 5 μ M of Betoptic S® treated under hypoxic conditions (hypoxia, Betoptic S®). After incubation for 4, 8, 12 and 24 hours, HO-1 expression was analyzed using Western blotting.
Results
Cell viability significantly increased in RGC-5 cells treated with Betoptic S® compared with other antiglaucoma agents. Increased levels of HO-1 expression indicate its relevance in cell viability. Furthermore, increased RGC-5 cell viability by Betoptic S® was significantly reduced in the HO-1 inhibitor ZnPP-treated group.
Go to : 
References
1. Hirooka K, Kelly ME, Baldridge WH. . Suppressive actions of betaxolol on ionic currents in retinal ganglion cells may explain its neuroprotective effects. Exp Eye Res. 2000; 70:611–21.

2. Kobayashi H, Kobayashi K, Okinami S. Randomized clinical trial of topical betaxolol for persistent macular edema after vitrectomy and epiretinal membrane removal. Am J Ophthalmol. 2003; 136:244–51.

3. Chidlow G, Melena J, Osborne NN. Betaxolol, a beta(1)-a-drenoceptor antagonist, reduces Na(+) influx into cortical synapto-somes by direct interaction with Na(+) channels: comparison with other beta-adrenoceptor antagonists. Br J Pharmacol. 2000; 130:759–66.
4. Kobayashi T, Mori Y. Ca2+ channel antagonists and neuro-protection from cerebral ischemia. Eur J Pharmacol. 1998; 363:1–15.

5. Cheon EW, Park CH, Kang SS. . Betaxolol attenuates retinal ischemia/reperfusion damage in the rat. Neuroreport. 2003; 14:1913–7.

6. Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968; 61:748–55.

7. Clark JE, Foresti R, Green CJ, Motterlini R. Dynamics of haem oxygenase-1 expression and bilirubin production in cellular pro-tection against oxidative stress. Biochem J. 2000; 348:Pt 3. 615–9.

8. Barañano DE. Wolosker H. Bae BI. . A mammalian iron ATPase induced by iron. J Biol Chem. 2000; 275:15166–73.

9. Peyton KJ, Reyna SV, Chapman GB. . Heme oxygen-ase-1-derived carbon monoxide is an autocrine inhibitor of vas-cular smooth muscle cell growth. Blood. 2002; 99:4443–8.

10. Otterbein LE, Bach FH, Alam J. . Carbon monoxide has an-ti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000; 6:422–8.

11. Otterbein LE, Zuckerbraun BS, Haga M. . Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft re-jection and with balloon injury. Nat Med. 2003; 9:183–90.

12. Endo S, Tomita H, Ishiguro S, Tamai M. Effect of betaxolol on as-partate aminotransferase activity in hypoxic rat retina in vitro. Jpn J Pharmacol. 2002; 90:121–4.

13. Nagata T, Ueno S, Morita H. . Direct inhibition of N-meth-yl-D-aspartate (NMDA)-receptor function by antiglaucomatous beta-antagonists. J Pharmacol Sci. 2008; 106:423–34.
14. Melena J, Osborne NN. Metipranolol attenuates lipid peroxidation in rat brain: a comparative study with other antiglaucoma drugs. Graefes Arch Clin Exp Ophthalmol. 2003; 241:827–33.

15. Bach FH. Heme oxygenase-1 as a protective gene. Wien Klin Wochenschr. 2002; 114:Suppl. 4:1–3.
16. Koriyama Y, Nakayama Y, Matsugo S, Kato S. Protective effect of lipoic acid against oxidative stress is mediated by Keap1/Nrf2- dependent heme oxygenase-1 induction in the RGC-5 cellline. Brain Res. 2013; 1499:145–57.
17. Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988; 2:2557–68.

18. McCoubrey WK Jr. Huang TJ. Maines MD. Isolation and charac-terization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Biochem. 1997; 247:725–32.

19. Himori N, Maruyama K, Yamamoto K. . Critical neuro-protective roles of heme oxygenase-1 induction against axonal in-jury-induced retinal ganglion cell death. J Neurosci Res. 2014; 92:1134–42.

20. Osborne NN, Ji D, Majid AS. . Glutamate oxidative injury to RGC-5 cells in culture is necrostatin sensitive and blunted by a hy-drogen sulfide (H2S)-releasing derivative of aspirin (ACS14). Neurochem Int. 2012; 60:365–78.

21. McCoubrey WK Jr. Maines MD. The structure, organization and differential expression of the gene encoding rat heme oxygenase-2. Gene. 1994; 139:155–61.

22. Kikuchi G, Yoshida T, Noguchi M. Heme oxygenase and heme degradation. Biochem Biophys Res Commun. 2005; 338:558–67.

23. Wood JP, Schmidt KG, Melena J. . The beta-adrenoceptor an-tagonists metipranolol and timolol are retinal neuroprotectants: comparison with betaxolol. Exp Eye Res. 2003; 76:505–16.
24. Yu ZK, Chen YN, Aihara M. . Effects of beta-adrenergic re-ceptor antagonists on oxidative stress in purified rat retinal gan-glion cells. Mol Vis. 2007; 13:833–9.
Go to : 
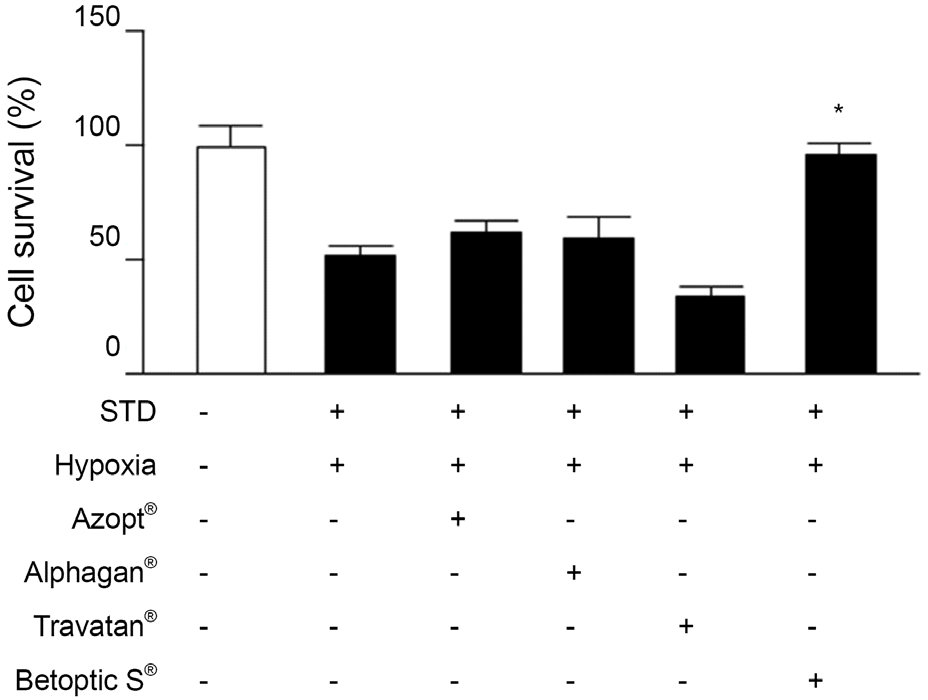 | Figure 1.Comparison of retinal ganglion cell (RGC-5) surviv-al rates with anti-glaucomatous agent after 48 hours. Addition of 5 μ M of betaxolol hydrochloride increased cellular survival (-STD: before preconditioning; +STD: after precondition-ing). STD = stauroporine; Azopt® = brinzolamide; Alphagan®= brimonidine tartrate; Travatan® = travopost; Betoptic S® = betaxolol hydrochloride. * p < 0.05 by student’s paired t-test. |
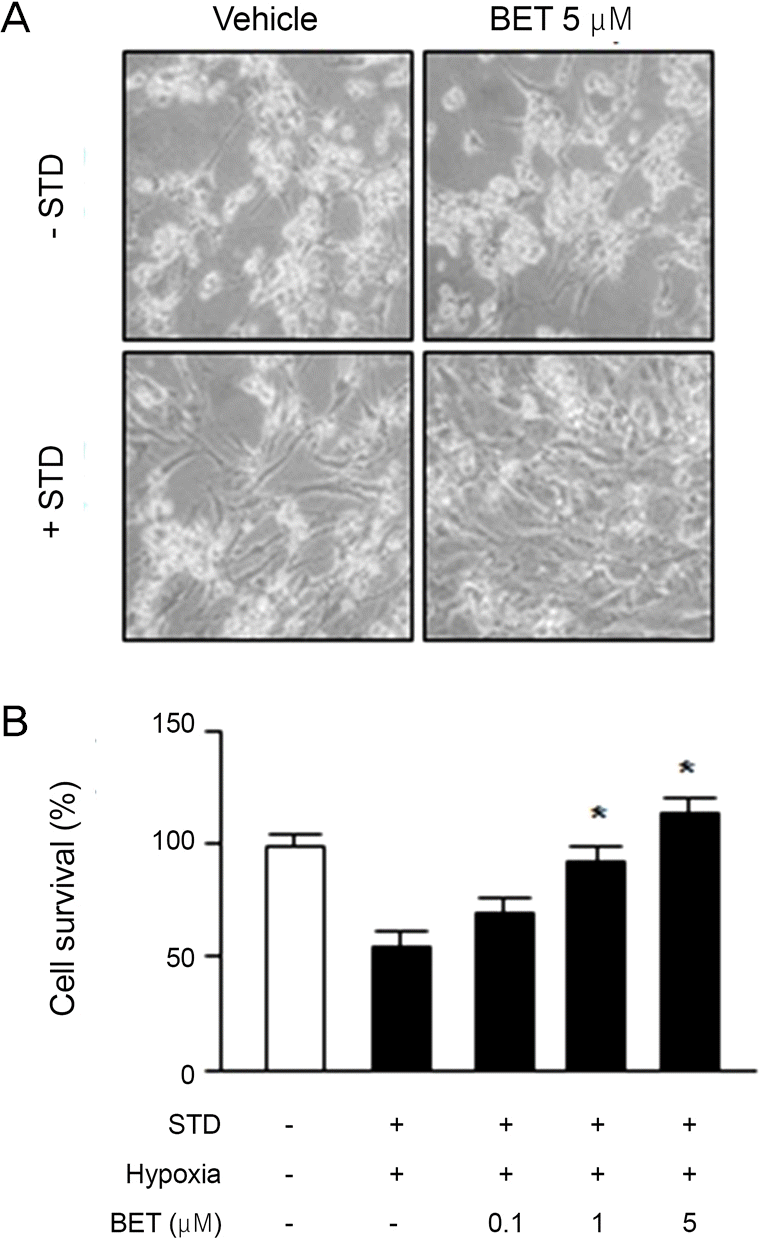 | Figure 2.Survival rates and microscopic finding of retinal gan-glion cells compared to BET concentraion. (A) Control and BET 5 μ M-treated group cultured for 48 hours and then taken by an op-tical microscope magnification 40 times without stain (-STD: be-fore preconditioning; +STD: after preconditioning). (B) Effect of BET on the survival of retinal ganglion cell (RGC-5) compared to BET concentration (Hypoxia: 5% CO2, 1% O2). STD = staur-oporine; BET = Betoptic S®. * p < 0.05 by student’s paired t-test. |
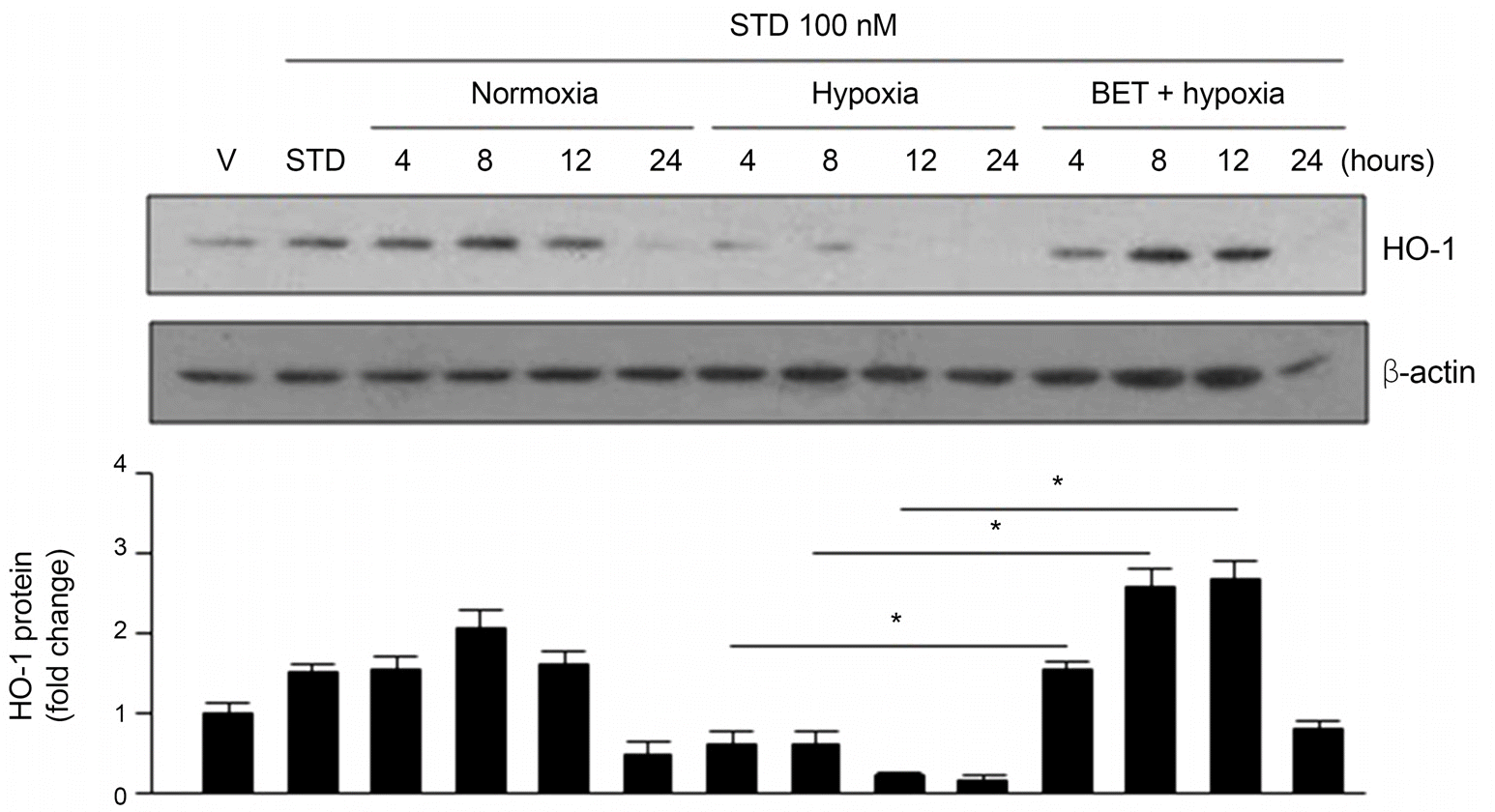 | Figure 3.Expression of HO-1 in accordance with the period in control group, hypoxia group, hypoxia and BET-treated group. Histogram presents the ratio of HO-1/β-actin expression. Western blotting was analyzed by the Image J software (Normoxia: 5% CO2, 20% O2; Hypoxia: 5% CO2, 1% O2). STD = stauroporine; BET = Betoptic S® 5 μ M; HO-1 = heme oxygenase-1. * p < 0.05 by student’s unpaired t-test. |
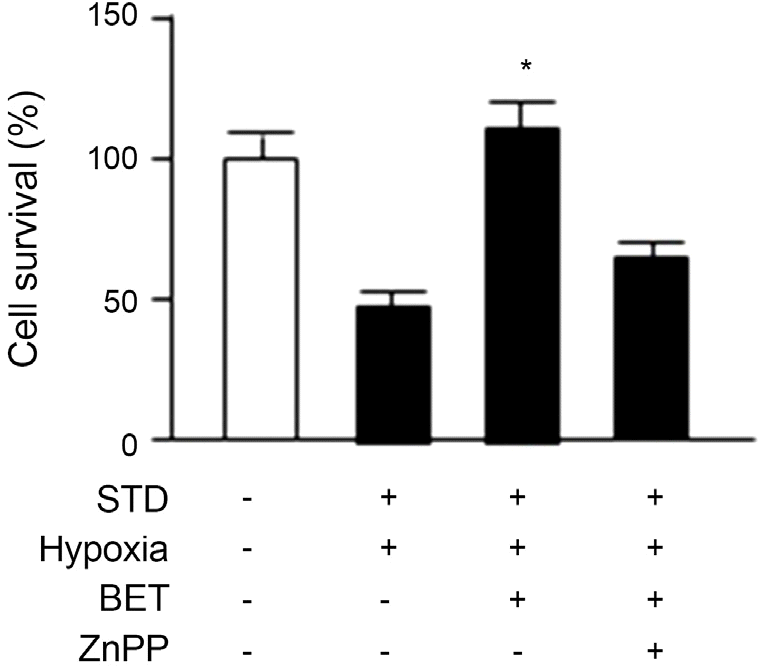 | Figure 4.Effect of HO-1 inhibitor on cell survival rates after ad-dition of BET. When compared to non-addition of HO-1 in-hibitor (ZnPP), Increased retinal ganglion cell (RGC-5) due to BET neuroprotective effect was reduced after addition of HO-1 inhibitor (ZnPP; -STD, – Hypoxia, -BET and – ZnPP: before preconditioning; +STD, +Hypoxia, -BET and -ZnPP: just af-ter the preconditioning). STD = stauroporine; BET = Betoptic S®; ZnPP = zinc protoporphyrin 10 μ M; HO-1 = heme oxy-genase-1. * p < 0.05 by student’s paired t-test. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download