Abstract
Purpose
To assess the agreement and compare the performance of glaucoma diagnosis of peripapillary retinal nerve fiber layer (RNFL) thickness measurements between two different spectraldomain optical coherence tomography (SD-OCT) devices.
Methods
Eighty nine eyes of 56 patients with glaucoma and 42 eyes of 25 healthy individuals were imaged with Cirrus and Spectralis OCT in a single visit. Agreement between RNFL thickness measurements was assessed using intraclass coefficient (ICC) and Bland-Altman plots. The discriminating abilities of the two techniques for detection of glaucoma were compared by the area under the receiver operating characteristic curves (AUC) for quadrants and average RNFL thickness.
Results
ICC values for agreement between both instruments were good for quadrants and average RNFL thickness (all ≥ 0.81). However, Spectralis OCT measurements were significantly greater than Cirrus OCT for temporal quadrant (difference = 4.27 µm in normal group, 3.91 µm in glaucoma group, p < 0.001 for both). The RNFL thickness parameter with the largest AUCs was the average RNFL thickness for the Spectralis OCT and the Cirrus OCT (0.85 vs. 0.87, p = 0.30). The pairwise comparison among the receiver operating characteristic curves showed no statistical difference for all parameters.
Conclusions
Although Spectralis OCT measurements were significantly greater than Cirrus OCT for temporal quadrant, agreement of RNFL measurement between both the devices was generally good and there was no statistically significant difference in the performance of glaucoma diagnosis between both instruments.
Go to : 
References
2. Kerrigan-Baumrind LA, Quigley HA, Pease ME, et al. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. 2000; 41:741–8.
3. Wollstein G, Kagemann L, Bilonick RA, et al. Retinal nerve fibre layer and visual function loss in glaucoma: the tipping point. Br J Ophthalmol. 2012; 96:47–52.

4. Shin JW, Lee WJ, Uhm KB. Comparison of diagnostic ability of 3D and stratus optical coherence tomography in early glaucoma. J Korean Ophthalmol Soc. 2012; 53:652–61.

5. Lee J, Song IS, Kim YJ, et al. Comparison of retinal nerve fiber layer thickness measured by spectraldomain and time-domain abdominalal coherence tomography. J Korean Ophthalmol Soc. 2012; 53:103–10.
6. Han KE, Jun RM, Choi KR. Comparison of RNFL thickness abdominal by two different kind of OCT in NTG patients. J Korean Ophthalmol Soc. 2009; 50:1853–9.
7. Lee Y, Sung KR, Hong JT, Na JH. Glaucoma diagnostic abdominal of macular and retinal nerve fiber layer by spectraldomain optical coherence tomography. J Korean Ophthalmol Soc. 2010; 51:1250–7.
8. Kim NR, Lee ES, Seong GJ, et al. Spectral-domain optical abdominal tomography for detection of localized retinal nerve fiber layer defects in patients with open-angle glaucoma. Arch Ophthalmol. 2010; 128:1121–8.
9. Hwang YH, Kim YY, Kim HK, Sohn YH. Ability of cirrus high-definition spectraldomain optical coherence tomography clock-hour, deviation, and thickness maps in detecting photographic retinal nerve fiber layer abnormalities. Ophthalmology. 2013; 120:1380–7.

10. Na JH, Lee KS, Lee JR, et al. The glaucoma detection capability of spectraldomain OCT and GDx-VCC deviation maps in early abdominal patients with localized visual field defects. Graefes Arch Clin Exp Ophthalmol. 2013; 251:2371–82.
11. Faghihi H, Hajizadeh F, Hashemi H, Khabazkhoob M. Agreement of two different spectral domain optical coherence tomography abdominal for retinal nerve fiber layer measurements. J Ophthalmic Vis Res. 2014; 9:31–7.
12. Valverde-Megías A, Martinez-de-la-Casa JM, Serrador-García M, et al. Clinical relevance of foveal location on retinal nerve fiber layer thickness using the new FoDi software in spectralis optical coherence tomography. Invest Ophthalmol Vis Sci. 2013; 54:5771–6.

13. Sung KR, Kim DY, Park SB, Kook MS. Comparison of retinal nerve fiber layer thickness measured by Cirrus HD and Stratus abdominalal coherence tomography. Ophthalmology. 2009; 116:1264–70. 1270.e1.
14. Leite MT, Rao HL, Zangwill LM, et al. Comparison of the abdominal accuracies of the Spectralis, Cirrus, and RTVue optical abdominal tomography devices in glaucoma. Ophthalmology. 2011; 118:1334–9.
15. Park HY, Lee K, Park CK. Optic disc torsion direction predicts the location of glaucomatous damage in normal-tension glaucoma abdominals with myopia. Ophthalmology. 2012; 119:1844–51.
16. Leite MT, Rao HL, Weinreb RN, et al. Agreement among abdominal-domain optical coherence tomography instruments for abdominal retinal nerve fiber layer thickness. Am J Ophthalmol. 2011; 151:85–92.e1.
17. Patel NB, Wheat JL, Rodriguez A, et al. Agreement between abdominall nerve fiber layer measures from Spectralis and Cirrus spectral domain OCT. Optom Vis Sci. 2012; 89:E652–66.
18. Knighton RW, Qian C. An optical model of the human retinal nerve fiber layer: implications of directional reflectance for variability of clinical measurements. J Glaucoma. 2000; 9:56–62.

19. Leung CK, Cheung CY, Weinreb RN, et al. Retinal nerve fiber abdominal imaging with spectraldomain optical coherence tomography: a variability and diagnostic performance study. Ophthalmology. 2009; 116:1257–63. 1263.e1–2.
20. Cho JW, Sung KR, Hong JT, et al. Detection of glaucoma by abdominal domain-scanning laser ophthalmoscopy/optical coherence abdominal (SD-SLO/OCT) and time domain optical coherence tomography. J Glaucoma. 2011; 20:15–20.
21. Park SB, Sung KR, Kang SY, et al. Comparison of glaucoma abdominal capabilities of Cirrus HD and Stratus optical coherence tomography. Arch Ophthalmol. 2009; 127:1603–9.
22. Kanamori A, Nakamura M, Escano MF, et al. Evaluation of the glaucomatous damage on retinal nerve fiber layer thickness abdominal by optical coherence tomography. Am J Ophthalmol. 2003; 135:513–20.
23. Medeiros FA, Zangwill LM, Bowd C, et al. Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness abdominals for glaucoma detection using optical coherence tomography. Am J Ophthalmol. 2005; 139:44–55.
Go to : 
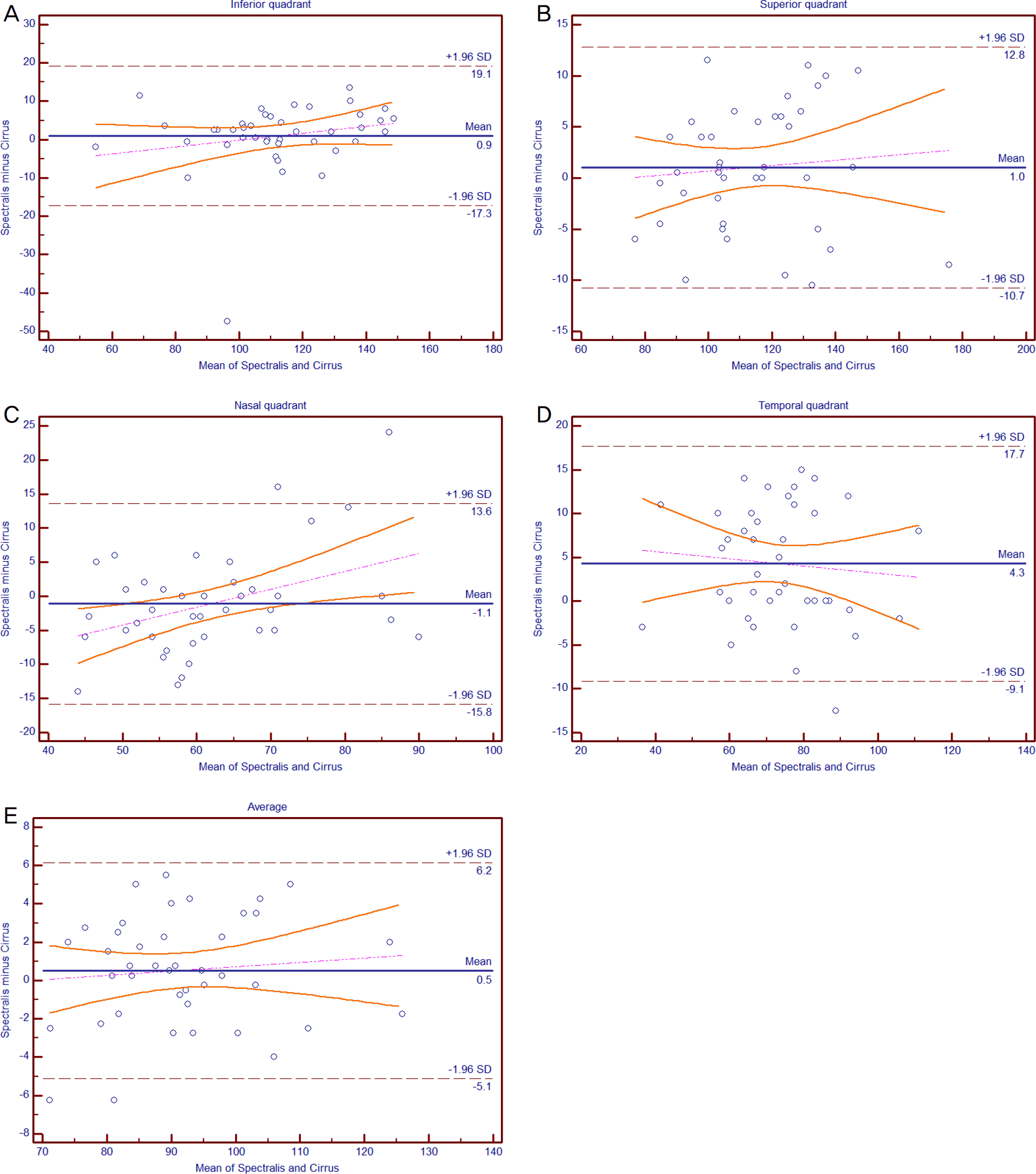 | Figure 1.Bland-Altman plots of agreement between Spectralis and Cirrus optical coherence tomography in normal eyes. These plots are generated by plotting the difference between the measurements against the average of the measurements. The solid line represents the trend line and the dashed lines represent the mean bias and the 95% limits of agreement. (A) Inferior, (B) superior, (C) nasal, (D) temporal quadrant, and (E) average retinal nerve fiber layer thickness. SD = standard deviation. |
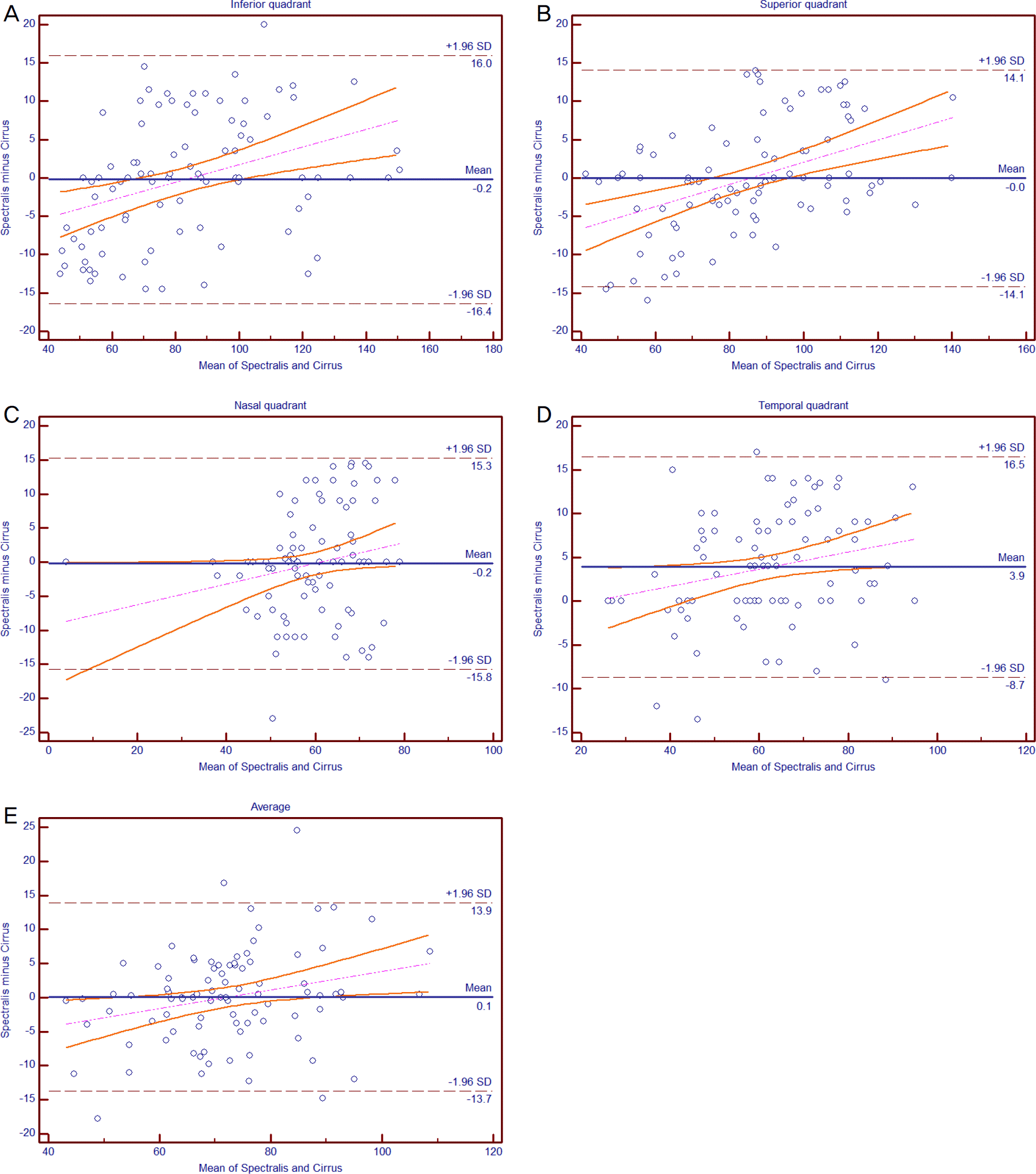 | Figure 2.Bland-Altman plots of agreement between Spectralis and Cirrus optical coherence tomography in patients with open-angle glaucoma. (A) Inferior, (B) superior, (C) nasal, (D) temporal quadrant, and (E) average retinal nerve fiber layer thickness. SD = standard deviation. |
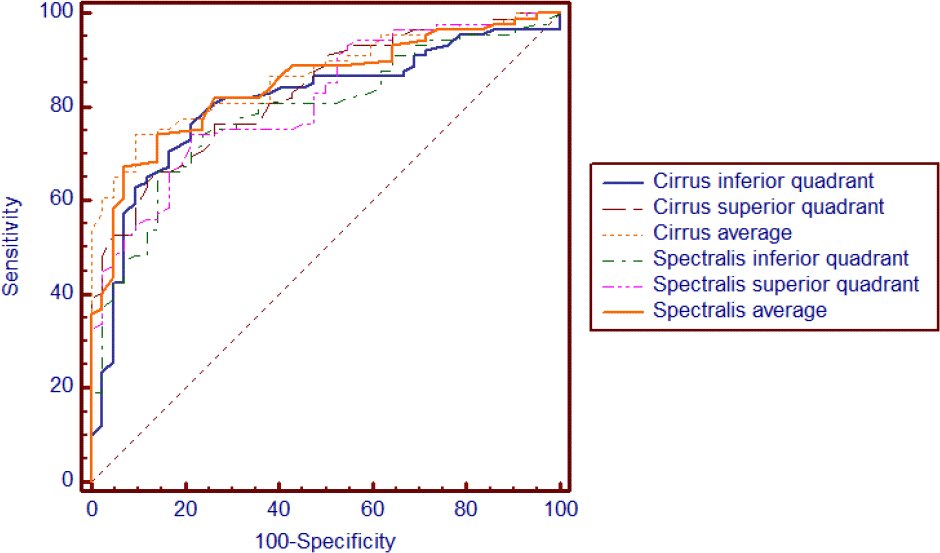 | Figure 3.The receiver operating characteristic curve of average, inferior and superior quadrant parapapillary retinal nerve fiber layer thickness for discrimination between normal and glaucomatous eyes. Sensitivities and specificities were described as percentage (%). |
Table 1.
Demographic and clinical characteristics of the patients enrolled in the study
| Normal | Glaucoma | p-value | |
|---|---|---|---|
| No. of eyes (patients) | 42 (25) | 89 (56) | |
| Age (years) | 42.1 ± 16.7 | 56.8 ± 15.1 | 0.128* |
| Sex (male/female) | 25/17 | 45/44 | 0.074† |
| Mean deviation (dB) | –1.2 ± 1.5 | –7.8 ± 5.7 | <0.001‡ |
| Pattern standard deviation (dB) | 1.7 ± 0.4 | 7.2 ± 5.0 | 0.030‡ |
| Central corneal thickness (μ m) | 550.4 ± 24.9 | 547.8 ± 35.5 | 0.011‡ |
| Spherical equivalent (diopter) | –3.6 ± 3.9 | –2.1 ± 3.4 | 0.092‡ |
| Axial length (mm) | 24.8 ± 1.9 | 24.5 ± 1.7 | 0.150‡ |
| Torsion degree (o) | –2.5 ± 13.4 | –3.1 ± 15.1 | 0.634‡ |
| Tilt index | 1.2 ± 0.1 | 1.2 ± 0.1 | 0.850‡ |
Table 2.
Comparison of retinal nerve fiber layer thickness measured with Cirrus and Spectralis optical coherence tomography
| Normal | Glaucoma | |||||
|---|---|---|---|---|---|---|
| Cirrus | Spectralis | p-value* | Cirrus | Spectralis | p-value* | |
| Inferior (μ m) | 111.7 ± 21.0 | 112.7 ± 22.8 | 0.520 | 83.4 ± 25.1 | 83.2 ± 28.1 | 0.818 |
| Superior (μ m) | 113.4 ± 20.2 | 114.5 ± 20.7 | 0.265 | 85.4 ± 21.2 | 85.3 ± 24.4 | 0.982 |
| Nasal (μ m) | 62.5 ± 10.7 | 61.4 ± 13.7 | 0.346 | 59.8 ± 10.8 | 59.6 ± 12.4 | 0.790 |
| Temporal (μ m) | 71.5 ± 15.4 | 75.8 ± 14.8 | <0.001 | 60.5 ± 15.0 | 64.4 ± 16.4 | <0.001 |
| Average (μ m) | 91.2 ± 12.4 | 91.7 ± 12.7 | 0.245 | 72.3 ± 12.9 | 72.4 ± 14.6 | 0.887 |
Table 3.
Intraclass correlation coefficient of retina nerve fiber layer thickness measurement with Cirrus and Spectralis optical coherence tomography
Table 4.
The area under receiver operator characteristics curve (AUC) and confidence interval (CI) of retinal nerve fiber layer thickness in average and 4 quadrants for discrimination of glaucoma from normal eyes




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download