Abstract
Purpose
To compare the characteristics of optical coherence tomography in eyes with treatment-naïve typical neovascular age-related macular degeneration (typical nAMD), polypoidal choroidal vasculopathy (PCV), and retinal angiomatous proliferation (RAP).
Methods
One hundred fifty-three eyes newly diagnosed with exudative AMD were retrospectively collected. All study eyes were classified into three subtypes: typical nAMD, PCV, and RAP. Subfoveal choroidal thickness (SFCT) was measured using enhanced depth imaging optical coherence tomography (EDI-OCT). Central macular thickness (CMT) and other OCT features including intraretinal cystoid fluid and subretinal fluid were also evaluated in all eyes. SFCT, CMT and other OCT features were compared among the three subtypes of exudative AMD.
Go to : 
References
1. Wong TY, Chakravarthy U, Klein R, et al. The natural history and prognosis of neovascular age-related macular degeneration: a abdominalatic review of the literature and meta-analysis. Ophthalmology. 2008; 115:116–26.
2. Jager RD, Mieler WF, Miller JW. Age-related macular abdominal. N Engl J Med. 2008; 358:2606–17.
3. Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic abdominal choroidal vasculopathy (IPCV). Retina. 1990; 10:1–8.
4. Ciardella AP, Donsoff IM, Huang SJ, et al. Polypoidal choroidal vasculopathy. Surv Ophthalmol. 2004; 49:25–37.

5. Yannuzzi LA, Negrão S, Iida T, et al. Retinal angiomatous abdominal in age-related macular degeneration. Retina. 2001; 21:416–34.
6. Liu Y, Wen F, Huang S, et al. Subtype lesions of neovascular age-related macular degeneration in Chinese patients. Graefes Arch Clin Exp Ophthalmol. 2007; 245:1441–5.

7. Uyama M, Wada M, Nagai Y, et al. Polypoidal choroidal abdominal: natural history. Am J Ophthalmol. 2002; 133:639–48.
8. Lee WK, Kwon SI. Polypoidal choroidal vasculopathy. J Korean Ophthalmol Soc. 2000; 41:2573–84.
9. Lee PY, Lee WK. Treatments of stage 1 retinal angiomatous proliferation. J Korean Ophthalmol Soc. 2008; 49:442–9.

10. Wilde C, Patel M, Lakshmanan A, et al. The diagnostic accuracy of spectraldomain optical coherence tomography for neovascular age-related macular degeneration: a comparison with fundus abdominal angiography. Eye (Lond). 2015; 29:602–9. quiz 610.
11. Hariri A, Heussen FM, Nittala MG, Sadda SR. Optical coherence tomographic correlates of angiographic subtypes of occult abdominal neovascularization. Invest Ophthalmol Vis Sci. 2013; 54:8020–6.
12. Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectraldomain optical coherence tomography. Am J Ophthalmol. 2008; 146:496–500.

13. Mrejen S, Spaide RF. Optical coherence tomography: imaging of the choroid and beyond. Surv Ophthalmol. 2013; 58:387–429.

14. Chen TC, Cense B, Pierce MC, et al. Spectral domain optical abdominal tomography: ultra-high speed, ultra-high resolution ophthalmic imaging. Arch Ophthalmol. 2005; 123:1715–20.
15. Lee JY, Lee DH, Lee JY, Yoon YH. Correlation between subfoveal choroidal thickness and the severity or progression of nonexudative age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013; 54:7812–8.

16. Chung SE, Kang SW, Lee JH, Kim YT. Choroidal thickness in abdominal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 2011; 118:840–5.
17. Kim SW, Oh J, Kwon SS, et al. Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous abdominal, and polypoidal choroidal vasculopathy. Retina. 2011; 31:1904–11.
18. Manjunath V, Goren J, Fujimoto JG, Duker JS. Analysis of abdominal thickness in age-related macular degeneration using spectraldomain optical coherence tomography. Am J Ophthalmol. 2011; 152:663–8.
19. Fein JG, Branchini LA, Manjunath V, et al. Analysis of short-term change in subfoveal choroidal thickness in eyes with age-related macular degeneration using optical coherence tomography. abdominal Surg Lasers Imaging Retina. 2014; 45:32–7.

20. Seddon JM, Sharma S, Adelman RA. Evaluation of the clinical age-related maculopathy staging system. Ophthalmology. 2006; 113:260–6.

21. Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009; 147:811–5.

22. Schmidt-Erfurth U, Waldstein SM. A paradigm shift in imaging abdominal in neovascular age-related macular degeneration. Prog Retin Eye Res. 2016; 50:1–24.
23. Verteporfin Roundtable Participants. Guidelines for using abdominal (Visudyne) in photodynamic therapy for choroidal abdominal due to age-related macular degeneration and other causes: update. Retina. 2005; 25:119–34.
24. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–31.

25. Kim YH, Kim ES, Yu SY, Kwak HW. abdominal effect of abdominal bevacizumab for CNV secondary to age-related macular degeneration. J Korean Ophthalmol Soc. 2008; 49:1935–40.
26. Rosa RH Jr, Davis JL, Eifrig CW. Clinicopathologic reports, case reports, and small case series: clinicopathologic correlation of abdominal polypoidal choroidal vasculopathy. Arch Ophthalmol. 2002; 120:502–8.
27. Kim JH, Kim JR, Kang SW, et al. Thinner choroid and greater abdominal extent in retinal angiomatous proliferation than in typical abdominal age-related macular degeneration. Am J Ophthalmol. 2013; 155:743–9. 749.e1–2.
28. Cohen SY, Dubois L, Tadayoni R, et al. Prevalence of reticular pseudodrusen in age-related macular degeneration with newly abdominal choroidal neovascularisation. Br J Ophthalmol. 2007; 91:354–9.
29. Park KH, Song SJ, Lee WK, et al. The results of abdominal abdominal of age-related macular degeneration in Korea. J Korean abdominal Soc. 2010; 51:516–23.
31. Grunwald JE, Hariprasad SM, DuPont J. Effect of aging on foveo-lar choroidal circulation. Arch Ophthalmol. 1998; 116:150–4.

32. Rishi P, Rishi E, Mathur G, Raval V. Ocular perfusion pressure and choroidal thickness in eyes with polypoidal choroidal abdominal, wet-age-related macular degeneration, and normals. Eye (Lond). 2013; 27:1038–43.
33. Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. Am J Ophthalmol. 2010; 150:325–9.e1.

34. Schmidt-Erfurth U, Waldstein SM, Deak GG, et al. Pigment abdominal detachment followed by retinal cystoid degeneration leads to vision loss in treatment of neovascular age-related macular degeneration. Ophthalmology. 2015; 122:822–32.
35. Sato T, Suzuki M, Ooto S, Spaide RF. Multimodal imaging abdominal and multimodal vision testing in neovascular age-related abdominal degeneration. Retina. 2015; 35:1292–302.
36. McBain VA, Kumari R, Townend J, Lois N. Geographic atrophy in retinal angiomatous proliferation. Retina. 2011; 31:1043–52.

37. Shah VP, Shah SA, Mrejen S, Freund KB. Subretinal abdominal exudation associated with neovascular age-related abdominal degeneration. Retina. 2014; 34:1281–8.
38. Liakopoulos S, Ongchin S, Bansal A, et al. Quantitative optical abdominal tomography findings in various subtypes of neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008; 49:5048–54.
Go to : 
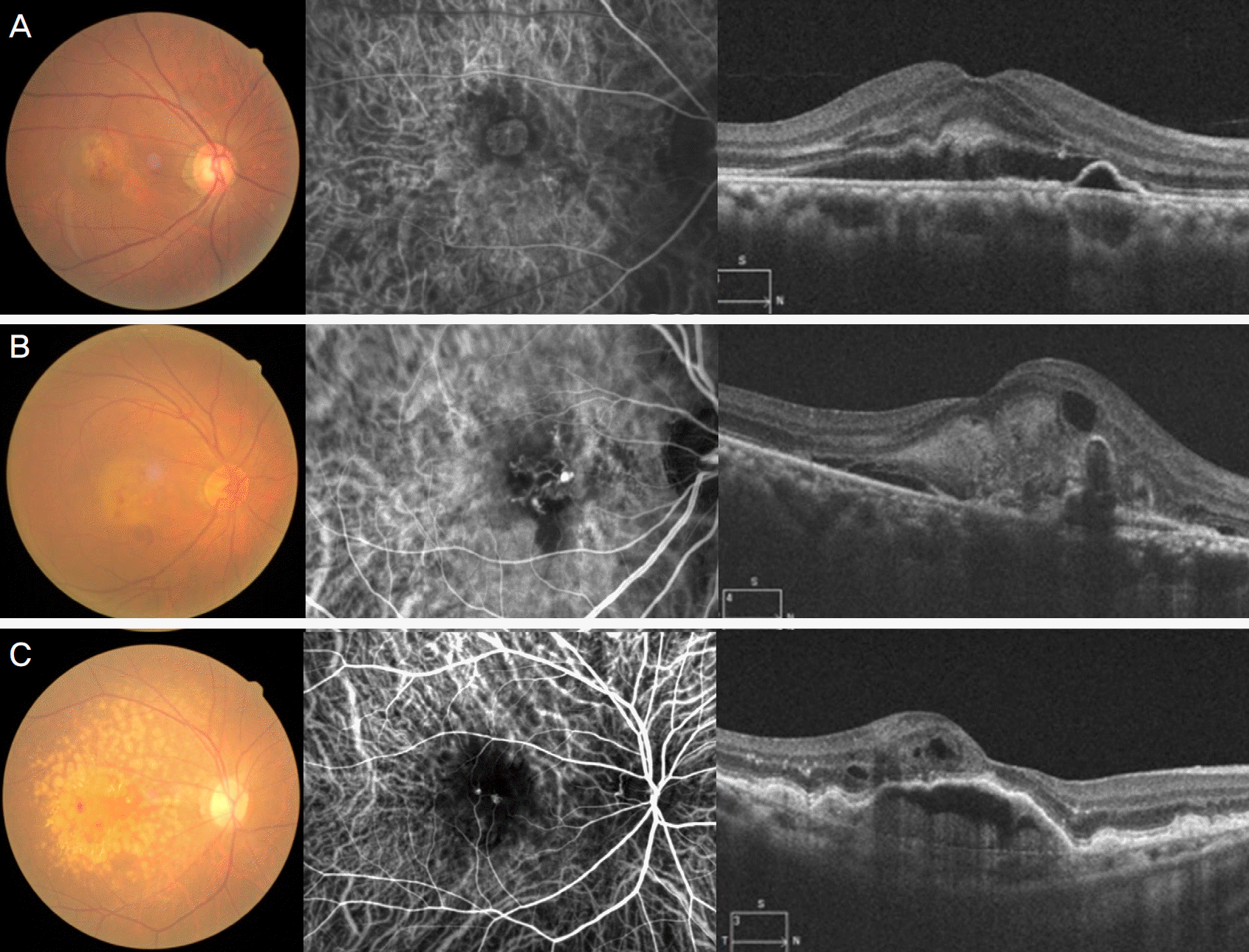 | Figure 1.Representative images of three subtypes of exudative age-related macular degeneration (AMD). Fundus photograph, indocyanine green angiography and optical coherence tomography images of typical neovascular AMD (A), polypoidal choroidal vasculopathy (B), and retinal angiomatous proliferation (C). |
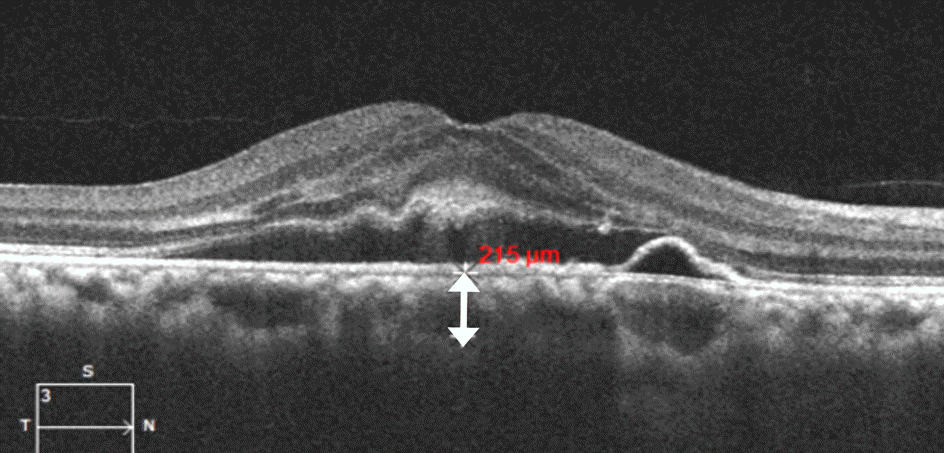 | Figure 2.Measurement of subfoveal choroidal thickness. The image was obtained using enhanced-depth imaging optical coherence tomography (OCT). Choroidal thickness (double-head arrow) was defined as the vertical distance drawn from the outer border of the retinal pigment epithelium to the inner border of the sclera using Cirrus High-Definition-OCT software. |
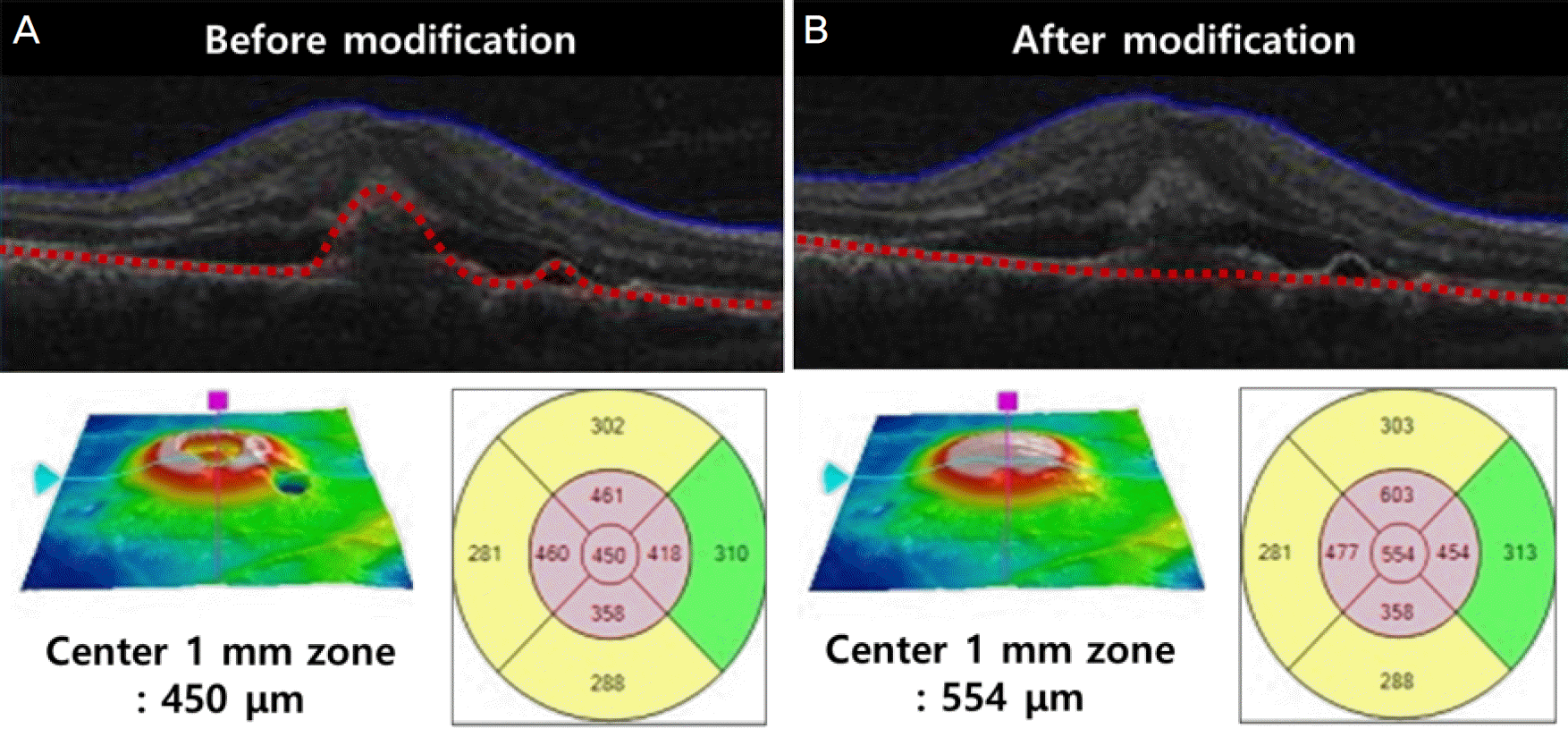 | Figure 3.Central macular thickness modification using a built-in segmentation-modifying tool of optical coherence tomography. Segmentation lines for retinal pigment epithelium (red dotted line) are determined automatically for each of the volume scans, and the central macular thickness is provided (A). Using the modifying tool, the cursor line on retinal pigment epithelium is moved to the level of Bruch's membrane. With this movement, the modified central macular thickness is determined (B). |
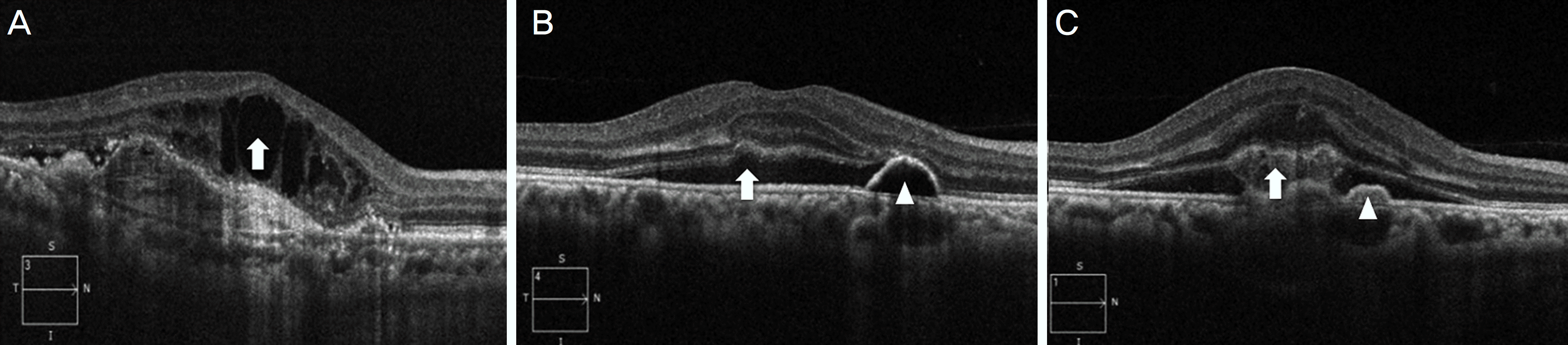 | Figure 4.Morphologic features of optical coherence tomography related to age-related macular degeneration. Intraretinal cystoid fluid is defined as round, hyporeflective cystic spaces within the neurosensory retina (A, arrow). Subretinal fluid is identified as non-reflective space between the posterior boundary of the neurosensory retina and the retinal pigment epithelium (B, arrow). Subretinal hyperreflective material appears as poorly defined, medium- to hyperreflective mass between neurosensory retina and retinal pigment epithelium (C, arrow). Pigment epithelial detachment is defined as a focal elevation of the reflective RPE band over an opti-cally clear or moderately reflective space (B, C; arrowhead). |
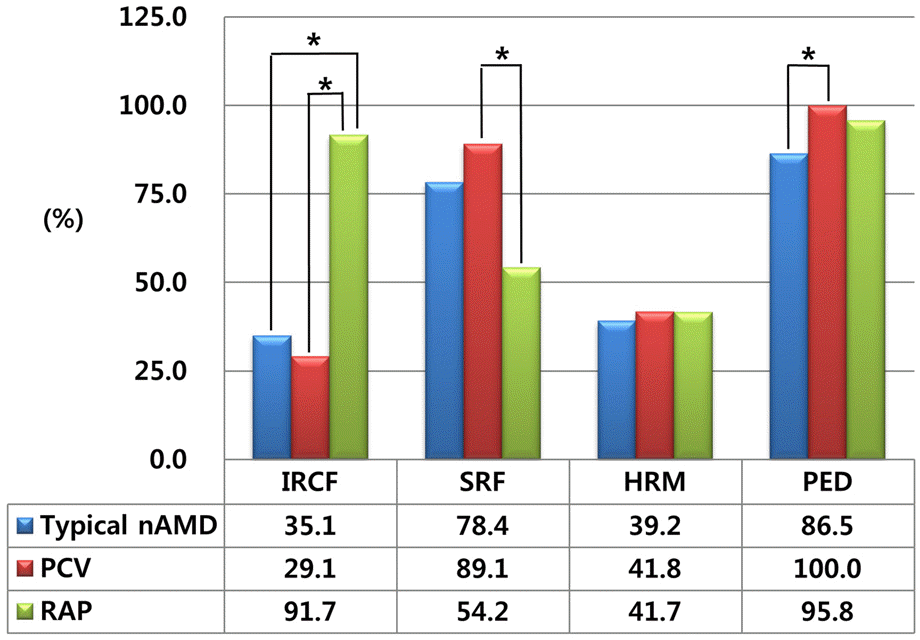 | Figure 5.Comparative incidence of morphological features on optical coherence tomography in each subtypes of exudative age-related macular degeneration. Accounting for a Bonferroni correction, p-values less than 0.017 were considered to indicate statistical significance. nAMD = neovascular agerelated macular degeneration; PCV = polypoidal choroidal vasculopathy; RAP = retinal angiomatous proliferation; IRCF = intraretinal cytroid fluid; SRF = subretinal fluid; HRM = subretinal hyperreflective material; PED = pigment epithelial detachment. * The frequency of morphological characteristics which have p-values less than 0.017 when compared with other subtype using Chi-square test. |
Table 1.
Demographic data of the patients
Table 2.
Comparison of baseline characteristics among three subtypes of exudative age-related macular degeneration
| Typical nAMD | PCV | RAP | p-value | |
|---|---|---|---|---|
| Number of eyes (n, %) | 74 (48.4%) | 55 (35.9%) | 24 (15.7%) | |
| Age (years) | 72.9 ± 8.5 | 69.8 ± 8.8 | 73.4 ± 7.9 | 0.086 |
| Sex (M:F) | 48:26 | 37:18 | 11:13 | 0.169 |
| Mean SE (diopters) | 0.18 ± 1.03 | 0.31 ± 0.82 | 0.40 ± 1.34 | 0.824 |
| Mean BCVA (log MAR) | 0.665 ± 0.490 | 0.657 ± 0.471 | 0.666 ± 0.323 | 0.993 |
| Mean SFCT (μ m) | 207.26 ± 57.16 | 253.76 ± 65.38 | 173.71 ± 39.83 | <0.001* |
| Mean CMT (μ m) | 442.39 ± 168.96 | 452.27 ± 135.87 | 564.46 ± 159.27 | 0.004* |
Values are presented as mean ± SD unless otherwise indicated. One way ANOVA for continuous variables; χ2 test for categorical variables. nAMD = neovascular age-related macular degeneration; PCV = polypoidal choroidal vasculopathy; RAP = retinal angiomatous proliferation; SE = spherical equivalent; BCVA = best corrected visual acuity; log MAR = logarithm of the minimum angle of resolution; SFCT = subfoveal choroidal thickness; CMT = central macular thickness.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download