Abstract
Purpose
Grape seed-derived polyphenols (GSPs) provide a concentrated source of polyphenols having antioxidant capacity. In this study we investigated the cytoprotective effect of GSP against oxidative stress-induced cell damage in cultured human reti-nal pigment epithelial (RPE) cells.
Methods
Cultured adult retinal pigment epithelium (ARPE)-19 cells were incubated with GSP from Vitis vinifera (0.1, 0.5, 1, 5 or 10 μ g/mL) for 24 hours and treated with hydrogen peroxide (H2 O2, 0.4 mM) for 24 hours to induce oxidative stress. Cell viability was measured using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Intracellular reactive oxygen species (ROS) was quantified using 2′,7′-dichlorofluorescein diacetate (DCF-DA) fluorescence.
Results
The percentage of viable RPE cells was significantly lower in cultures treated with H2 O2 0.4 mM than in control cultures. GSP significantly reduced H2 O2-induced cell death in a dose dependent manner. GSP at 0.1, 0.5, 1, 5 and 10 μ g/mL significantly reduced cell mortality due to the treatment with H2 O2. Intracellular ROS production increased significantly in cultures treated with H2 O2 0.4 mM compared with control. There was a significant dose-dependent decrease in intracellular ROS levels after treat-ment of RPE with GSP.
Go to : 
References
1. Devore EE, Kang JH, Breteler MM, Grodstein F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann Neurol. 2012; 72:135–43.

2. McCullough ML, Peterson JJ, Patel R. . Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am J Clin Nutr. 2012; 95:454–64.

3. Wang Y, Stevens VL, Shah R. . Dietary flavonoid and proan-thocyanidin intakes and prostate cancer risk in a prospective cohort of US men. Am J Epidemiol. 2014; 179:974–86.

4. Manach C, Scalbert A, Morand C. . Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004; 79:727–47.

5. Takikawa M, Inoue S, Horio F, Tsuda T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J Nutr. 2010; 140:527–33.

6. Vepsäläinen S, Koivisto H, Pekkarinen E. . Anthocyanin-en-riched bilberry and blackcurrant extracts modulate amyloid pre-cursor protein processing and alleviate behavioral abnormalities in the APP/PS1 mouse model of Alzheimer's disease. J Nutr Biochem. 2013; 24:360–70.

7. Wang J, Ferruzzi MG, Ho L. . Brain-targeted proanthocyanidin metabolites for Alzheimer's disease treatment. J Neurosci. 2012; 32:5144–50.

8. Dong X, Li Z, Wang W. . Protective effect of canolol from oxi-dative stress-induced cell damage in ARPE-19 cells via an ERK mediated antioxidative pathway. Mol Vis. 2011; 17:2040–8.
9. Li Z, Dong X, Liu H. . Astaxanthin protects ARPE-19 cells from oxidative stress via upregulation of Nrf2-regulated phase II enzymes through activation of PI3K/Akt. Mol Vis. 2013; 19:1656–66.
10. Hanneken A, Lin FF, Johnson J, Maher P. Flavonoids protect hu-man retinal pigment epithelial cells from oxidative-stress-induced death. Invest Ophthalmol Vis Sci. 2006; 47:3164–77.

11. Kil HK, Song YM, Chun K. The efficacy of vaccinium uliginosum for early age-related macula degeneration. J Korean Ophthalmol Soc. 2013; 54:1255–60.
12. Moini H, Rimbach G, Packer L. Molecular aspects of procyanidin biological activity: disease preventative and therapeutic potentials. Drug Metabol Drug Interact. 2000; 17:237–59.

13. Vigna GB, Costantini F, Aldini G. . Effect of a standardized grape seed extract on low-density lipoprotein susceptibility to oxi-dation in heavy smokers. Metabolism. 2003; 52:1250–7.

14. Gabetta B, Fuzzati N, Griffini A. . Characterization of proan-thocyanidins from grape seeds. Fitoterapia. 2000; 71:162–75.

15. Pieters R, Huismans DR, Leyva A, Veerman AJ. Adaptation of the rapid automated tetrazolium dye based (MTT) assay for chemo-sensitivity testing in childhood leukemia. Cancer Lett. 1988; 41:323–32.

16. Uddin S, Ahmad S. Dietary antioxidants protection against oxida-tive stress. Biochem Educ. 1995; 23:2–7.

17. Jerome-Morais A, Diamond AM, Wright ME. Dietary supple-ments and human health: for better or for worse? Mol Nutr Food Res. 2011; 55:122–35.
18. Schraermeyer U, Heimann K. Current understanding on the role of retinal pigment epithelium and its pigmentation. Pigment Cell Res. 1999; 12:219–36.

19. Cai J, Nelson KC, Wu M. . Oxidative damage and protection of the RPE. Prog Retin Eye Res. 2000; 19:205–21.

20. Lee JH, Kim JW. Effect of glucose on the production of reactive oxygen species in retinal pigment epithelial cells. J Korean Ophthalmol Soc. 2010; 51:276–81.

21. Murota K, Mitsukuni Y, Ichikawa M. . Quercetin-4'-glucoside is more potent than quercetin-3-glucoside in protection of rat in-testinal mucosa homogenates against iron ion-induced lipid peroxidation. J Agric Food Chem. 2004; 52:1907–12.

22. Kook D, Wolf AH, Yu AL. . The protective effect of quercetin against oxidative stress in the human RPE in vitro. Invest Ophthalmol Vis Sci. 2008; 49:1712–20.

23. Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms con-trolling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003; 43:233–60.

24. Tanito M, Masutani H, Kim YC. . Sulforaphane induces thio-redoxin through the antioxidant-responsive element and attenuates retinal light damage in mice. Invest Ophthalmol Vis Sci. 2005; 46:979–87.

25. King RE, Kent KD, Bomser JA. Resveratrol reduces oxidation and proliferation of human retinal pigment epithelial cells via ex-tracellular signal-regulated kinase inhibition. Chem Biol Interact. 2005; 151:143–9.

26. Singh S, Aggarwal BB. Activation of transcription factor NF-kap-pa B is suppressed by curcumin (diferuloylmethane) [corrected]. J Biol Chem. 1995; 270:24995–5000.
27. Li MH, Jang JH, Sun B, Surh YJ. Protective effects of oligomers of grape seed polyphenols against beta-amyloid-induced oxidative cell death. Ann N Y Acad Sci. 2004; 1030:317–29.
28. Zhang B, Osborne NN. Oxidative-induced retinal degeneration is attenuated by epigallocatechin gallate. Brain Res. 2006; 1124:176–87.

29. De Marchi U, Biasutto L, Garbisa S. . Quercetin can act either as an inhibitor or an inducer of the mitochondrial permeability transition pore: a demonstration of the ambivalent redox character of polyphenols. Biochim Biophys Acta. 2009; 1787:1425–32.

30. Wätjen W, Michels G, Steffan B. . Low concentrations of fla-vonoids are protective in rat H4IIE cells whereas high concen-trations cause DNA damage and apoptosis. J Nutr. 2005; 135:525–31.
31. Bouayed J, Bohn T. Exogenous antioxidants - double-edged swords in cellular redox state: Health beneficial effects at physio-logic doses versus deleterious effects at high doses. Oxid Med Cell Longev. 2010; 3:228–37.
32. Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related mac-ular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001; 119:1417–36.
33. Age-Related Eye Disease Study 2 Research Group Lutein + zeax-anthin and omega-3 fatty acids for age-related macular degener-ation: the Age-Related Eye Disease Study 2 (AREDS2) random-ized clinical trial. JAMA. 2013; 309:2005–15.
Go to : 
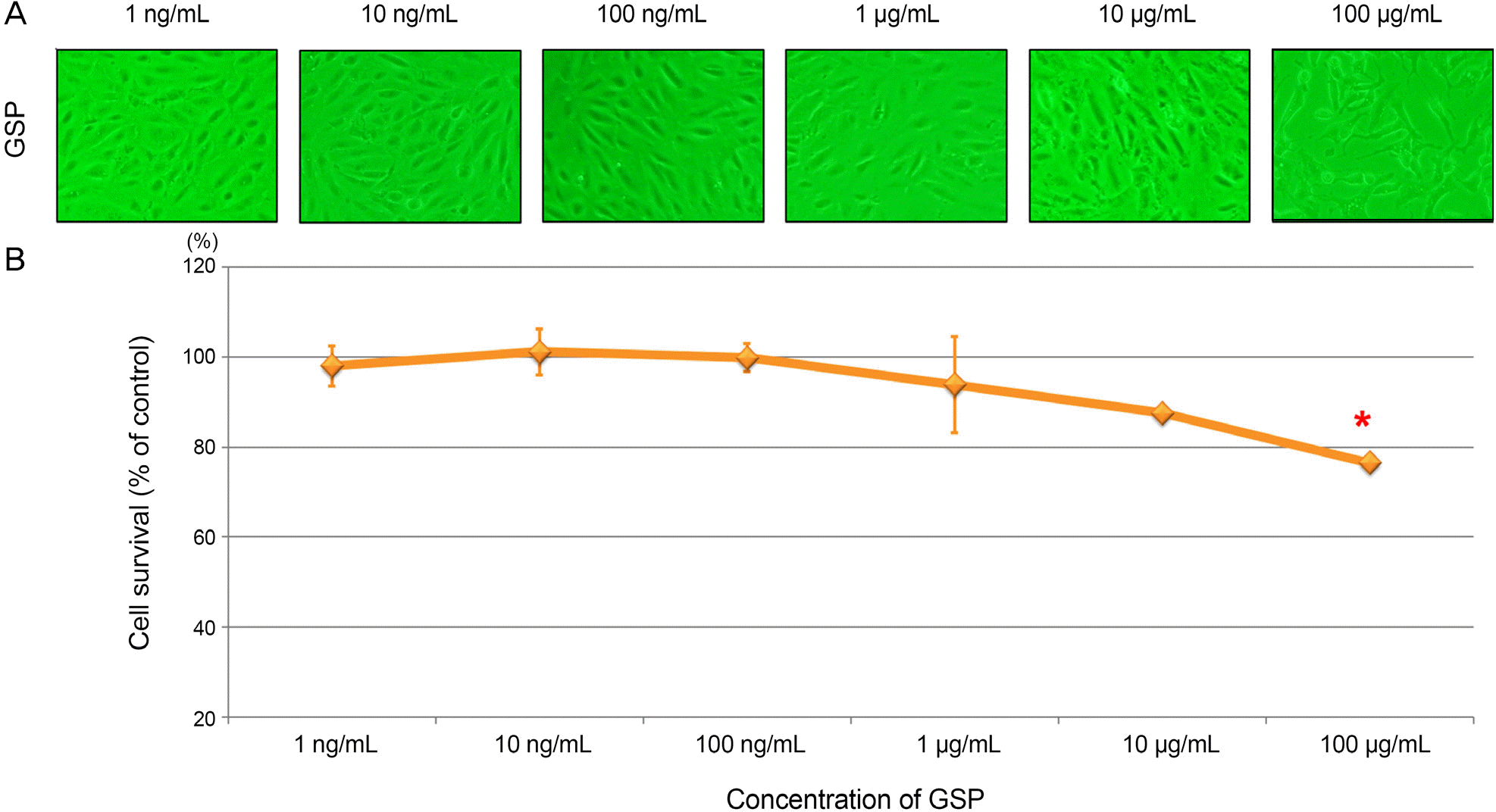 | Figure 1.Cytotoxic effect of grape seed-derived polyphenols (GSP). The ARPE-19 cells were incubated with different concen-tration of GSP for 24 hours. (A) GSP showed cytotoxicity at 100 μ g/mL. (B) The asterisk (*) indicates p < 0.05 versus untreated control. ARPE = adult retinal pigment epithelium. |
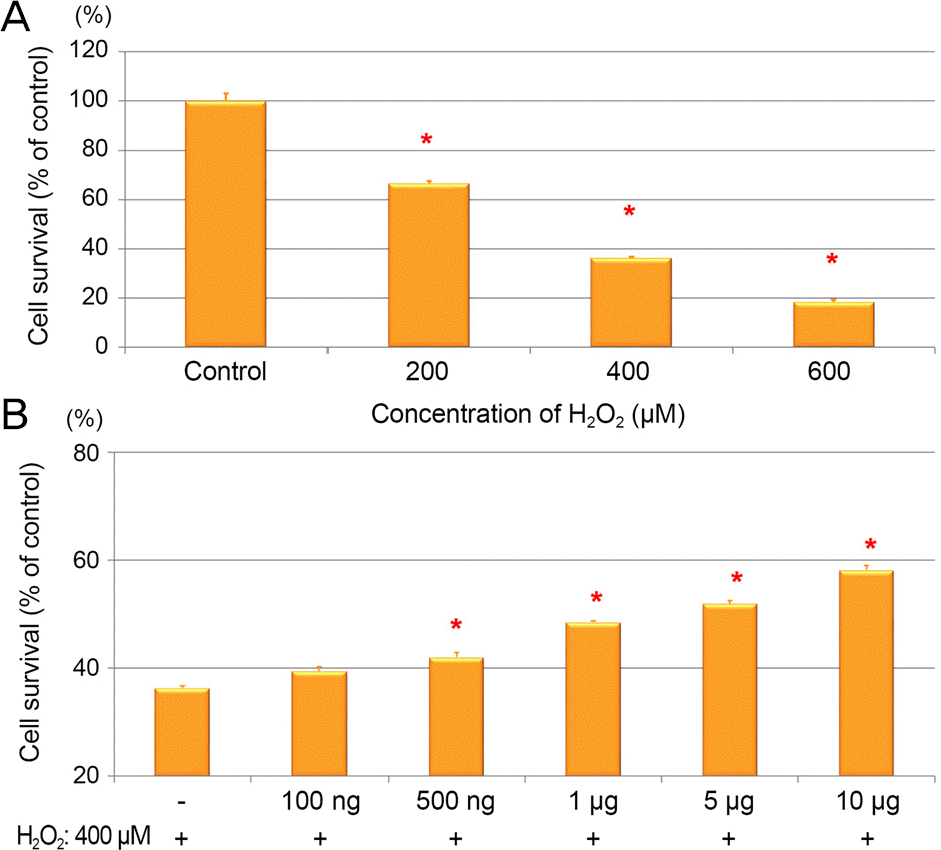 | Figure 2.Grape seed-derived polyphenols (GSP) protected against H2 O2 cell death in ARPE-19 cells. (A) The ARPE-19 cells were treated with H2 O2 at dose of 200, 400, and 600 μ M for 24 hours. (B) The cells were pretreated with GSP for 24 hours then exposed to H2 O2 (400 μ M) for 24 hours. Data was expressed as a percentage of the untreated control. The aster-isk (*) indicates p < 0.05 versus untreated control (A), or H2 O2-induced cells without GSP pretreatment (B). ARPE = adult retinal pigment epithelium. |
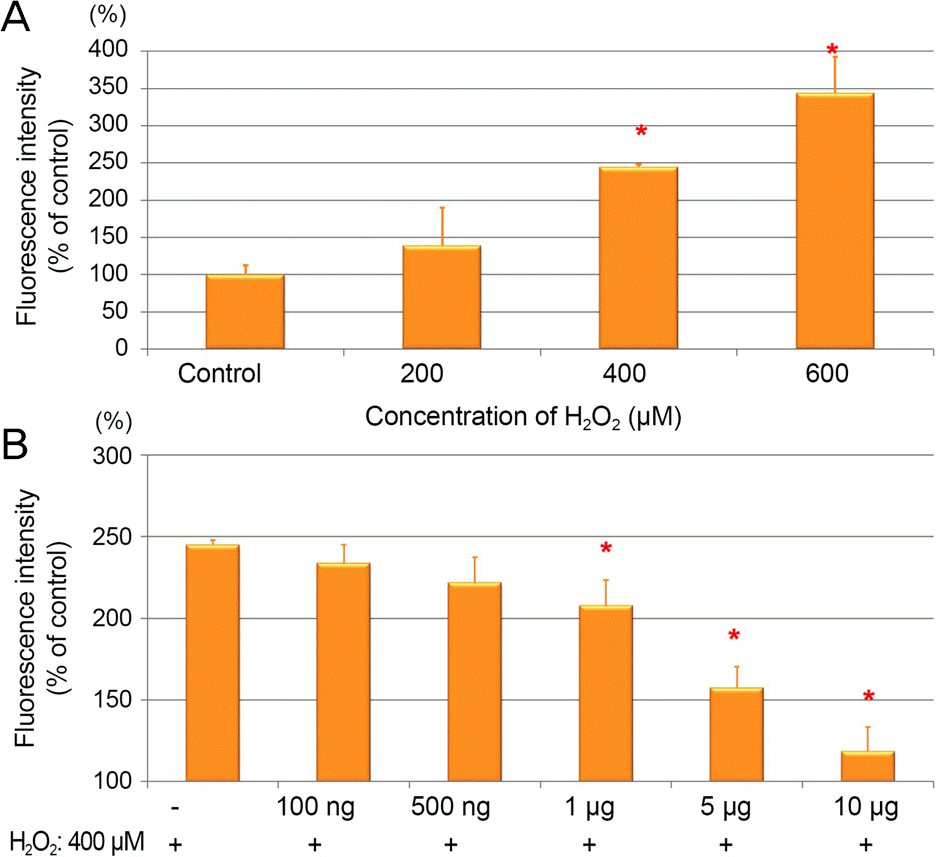 | Figure 3.Grape seed-derived polyphenols (GSP) reduced H2 O2-induced intracellular reactive oxygen species (ROS) generation in ARPE-19 cells. (A) The ARPE-19 cells were treated with H2 O2 at dose of 200, 400, and 600 μ M for 24 hours. (B) The cell were pretreated with GSP for 24 hours then treated with 400 μ M for 4 hours. The intracellular ROS was examined by a fluorescence spectrophotometer using DCF- DA. Data was expressed as a percentage of the untreated control. The asterisk (*) indicates p < 0.05 versus untreated control (A), or H2 O2-induced cells without GSP pretreatment (B). ARPE = adult retinal pigment epithelium; DCF-DA = 2′,7′-dichlorofluorescein diacetate. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download