Abstract
Purpose
To investigate the actual adherence to treatment with preservative-free dorzolamide-timolol fixed combination (DTFC) eyedrops of primary open-angle glaucoma (POAG) patients by counting the number of unused single-dose units of DTFC.
Methods
This study included 34 POAG patients newly prescribed with preservative-free DTFC eyedrops (formulated in sin-gle-dose units). The enrolled patients were asked to bring the unused DTFC units on their next visit after 2 weeks of treatment with DTFC. On their second visit, they were asked to complete a questionnaire regarding the self-reported adherence and the number of unused DTFC single-dose units was counted. The actual adherence (%) was calculated by dividing the expected number of used DTFC units by the actual number of used DTFC units. The correlation between the self-reported adherence and the measured adherence was assessed.
Results
Twenty-nine (93.5%) patients answered they adhered to the medication by more than 90% and 2 (6.5%) answered they instilled the eyedrops at 80-90% of the dosing schedule. However, after counting the unused DTFC single-dose units, 9 (29.0%) patients showed an actual adherence of <90%. Moreover, the actual adherence of 3 (9.7%) patients was <60%. Unexpectedly, 4 (12.9%) patients showed the actual adherence exceeding 100% (196%, 1 patient; 107-132%, 3 patients).
Conclusions
We demonstrated a large difference between the self-reported and the actual adherence to treatment by counting the unused single-dose units of eyedrops. Preservative-free topical anti-glaucoma medications (formulated in single-dose units) provide clinicians an opportunity to assess the actual adherence of glaucoma patients by counting the unused units of eyedrops. J Korean Ophthalmol Soc 2015;56(6):906-910
References
1. Kingman S. Glaucoma is second leading cause of blindness glo-bally. Bull World Health Organ. 2004; 82:887–8.
2. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120:701–13. discussion 829-30.
3. Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998; 126:498–505.
4. Friedman DS, Wilson MR, Liebmann JM, et al. An evidence-based assessment of risk factors for the progression of ocular hypertension and glaucoma. Am J Ophthalmol. 2004; 138(3 Suppl):S19–31.

6. Ashburn FS Jr, Goldberg I, Kass MA. Compliance with ocular therapy. Surv Ophthalmol. 1980; 24:237–48.

7. DiMatteo MR. Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004; 42:200–9.
8. Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol. 2008; 53(Suppl 1):S57–68.

9. Kist K. Basis of quantitative perimetry. Anderson DR, Patella VM, editors. Automated static perimetry. 2nd ed.St. Louis: Mosby;1998. chap. 2.
10. Feuer WJ. Glaucomatous visual field loss. Hodapp E, Parrish RK, Anderson DR, editors. Clinical decisions in glaucoma. 1st ed.St. Louis: Mosby;1993. chap. 2.
11. Beckers HJ, Schouten JS, Webers CA, et al. Side effects of com-monly used glaucoma medications: comparison of tolerability, chance of discontinuation, and patient satisfaction. Graefes Arch Clin Exp Ophthalmol. 2008; 246:1485–90.

12. Hahn SR. Patient-centered communication to assess and enhance patient adherence to glaucoma medication. Ophthalmology. 2009; 116(11 Suppl):S37–42.

13. Ahn DH, Lee YG, Hong YJ. Factors affecting compliance with prescribed eyedrops for glaucoma. J Korean Ophthalmol Soc. 1998; 39:2145–51.
Figure 1.
Number of patients complaining of uncomfortable side effects of preservative-free dorzolamide-timolol fixed combination.
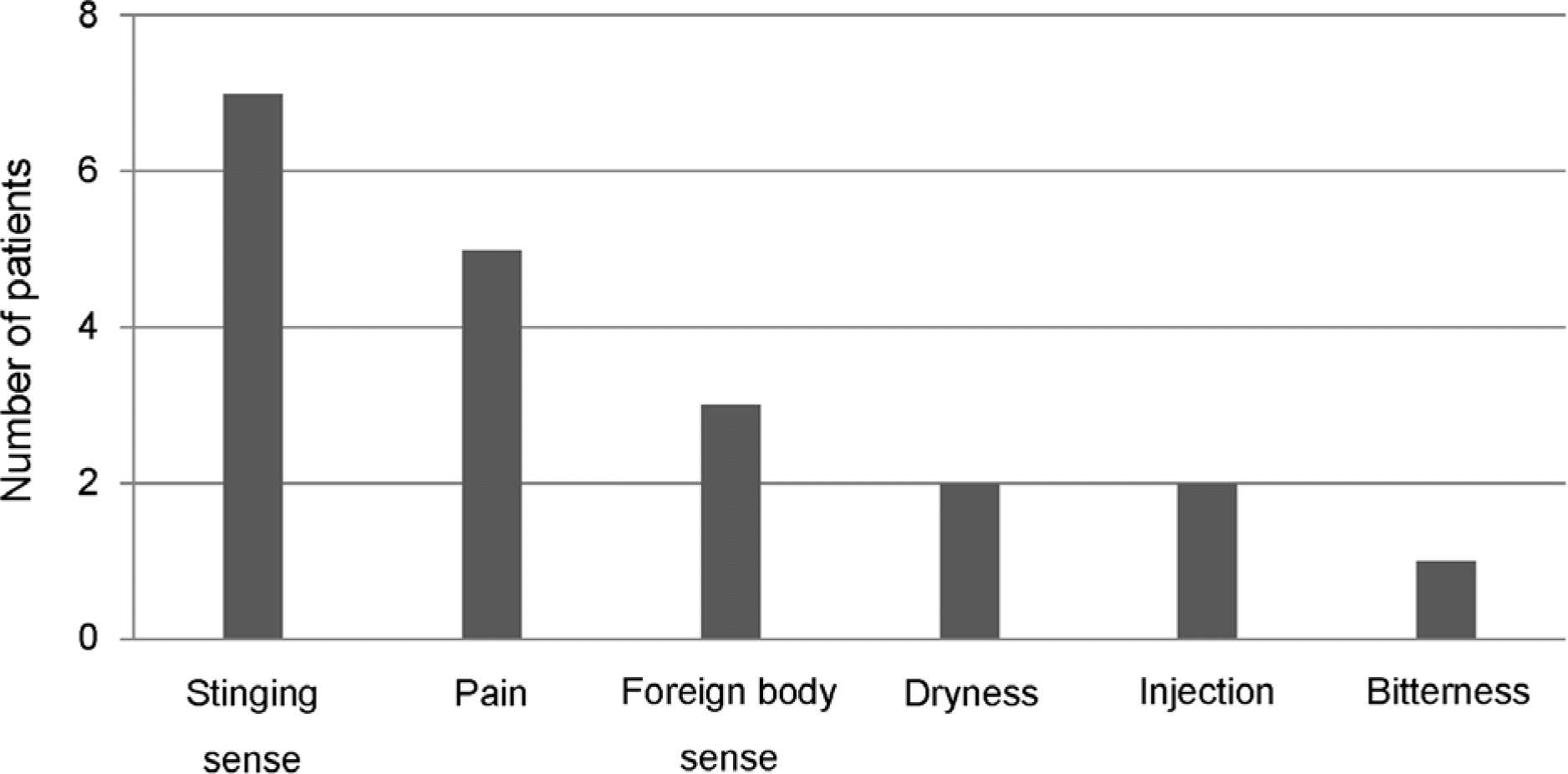
Figure 2.
Graph showing the percentage of prescribed doses taken by patients (x-axis) and the percentage of patients (y-axis).
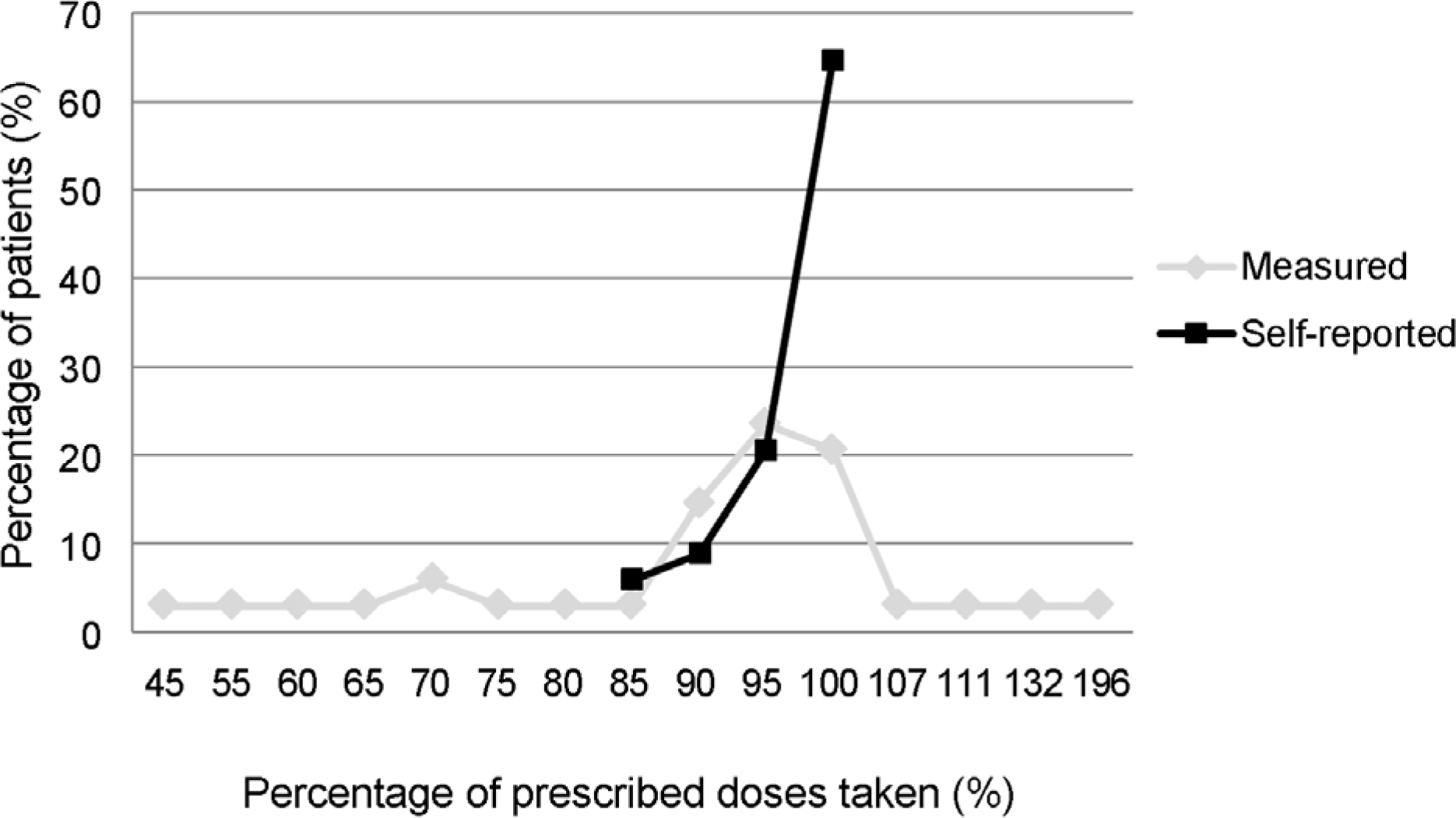
Figure 3.
Scatterplot showing the Spearman’s correlation between the self-reported adherence (x-axis) and the measured adherence (y-axis) (p=0.064).
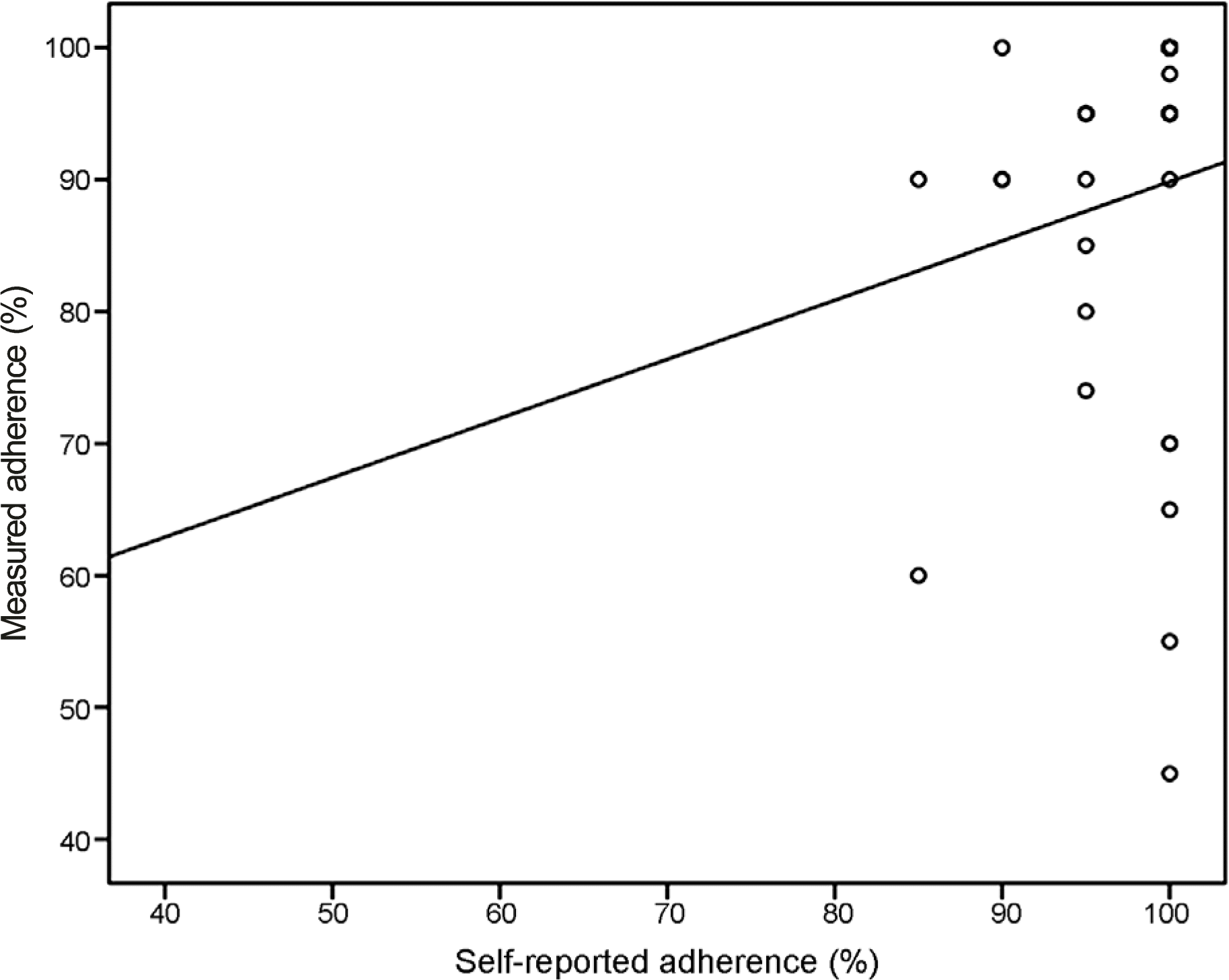
Table 1.
Patient characteristics




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download