1. Campochiaro PA, Hafiz G, Shah SM, et al. Ranibizumab for macular edema due to retinal vein occlusions: implication of VEGF as a critical stimulator. Mol Ther. 2008; 16:791–9.

2. Chen KH, Wu CC, Roy S, et al. Increased interleukin-6 in aqueous humor of neovascular glaucoma. Invest Ophthalmol Vis Sci. 1999; 40:2627–32.
3. Owen LA, Hartnett ME. Soluble mediators of diabetic macular edema: the diagnostic role of aqueous VEGF and cytokine levels in diabetic macular edema. Curr Diab Rep. 2013; 13:476–80.

4. Rogers S, McIntosh RL, Cheung N, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010; 117:313–9.e1.

5. Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol. 1996; 114:1243–7.

6. Rehak J, Rehak M. Branch retinal vein occlusion: pathogenesis, visual prognosis, and treatment modalities. Curr Eye Res. 2008; 33:111–31.

7. Lowder C, Belfort R Jr, Lightman S, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011; 129:545–53.

8. Freeman G, Matos K, Pavesio CE. Cystoid macular oedema in uveitis: an unsolved problem. Eye (Lond). 2001; 15(Pt 1):12–7.

9. Hunter RS, Lobo AM. Dexamethasone intravitreal implant for the treatment of noninfectious uveitis. Clin Ophthalmol. 2011; 5:1613–21.
10. Yeh PC, Ramanathan S. Latanoprost and clinically significant cystoid macular edema after uneventful phacoemulsification with intraocular lens implantation. J Cataract Refract Surg. 2002; 28:1814–8.

11. Arcieri ES, Santana A, Rocha FN, et al. Blood-aqueous barrier changes after the use of prostaglandin analogues in patients with pseudophakia and aphakia: a 6-month randomized trial. Arch Ophthalmol. 2005; 123:186–92.
12. Boyer DS, Yoon YH, Belfort R Jr, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014; 121:1904–14.

13. Haller JA, Bandello F, Belfort R Jr, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011; 118:2453–60.
14. Leopold IH. Update on antibiotics in ocular infections. Am J Ophthalmol. 1985; 100:134–40.

15. Zalewski D, Raczyńska D, Raczyńska K. Five-month observation of persistent diabetic macular edema after intravitreal injection of Ozurdex implant. Mediators Inflamm. 2014; 2014:364143.

16. Guigou S, Hajjar C, Parrat E, et al. Multicenter Ozurdex(R) assessment for diabetic macular edema: MOZART study. J Fr Ophtalmol. 2014; 37:480–5.
17. Furino C, Boscia F, Recchimurzo N, et al. Intravitreal dexamethasone implant for macular edema following uncomplicated phacoemulsification. Eur J Ophthalmol. 2014; 24:387–91.

18. Gallego-Pinazo R, Marín-Lambíes C, Marín-Olmos F, et al. Intravitreal dexamethasone as an enhancer for the anti-VEGF treatment in neovascular ARMD: recovering an old ally. Arch Soc Esp Oftalmol. 2010; 85:79–80.

19. Latronico ME, Rigante D, Caso F, et al. Bilateral dexamethasone intravitreal implant in a young patient with Vogt-Koyanagi-Harada disease and refractory uveitis. Clin Rheumatol. 2014 Apr 25; [Epub].

20. Kapoor KG, Wagner MG, Wagner AL. The sustained-release dexamethasone implant: expanding indications in vitreoretinal disease. Semin Ophthalmol. 2014 Mar 21; [Epub ahead of print].

21. Saatci AO, Doruk HC, Yaman A. Intravitreal dexamethasone implant (ozurdex) in coats' disease. Case Rep Ophthalmol. 2013; 4:122–8.

22. Haller JA, Bandello F, Belfort R Jr, et al. Randomized, sham-con-trolled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010; 117:1134–46.e3.

23. Becker B. Intraocular pressure response to topical corticosteroids. Invest Ophthalmol. 1965; 4:198–205.
24. Armaly MF. Statistical attributes of the steroid hypertensive response in the clinically normal eye. I. The demonstration of three levels of response. Invest Ophthalmol. 1965; 4:187–97.
25. Meyer LM, Schönfeld CL. Secondary glaucoma after intravitreal dexamethasone 0.7 mg implant in patients with retinal vein occlusion: a one-year follow-up. J Ocul Pharmacol Ther. 2013; 29:560–5.
26. Kubota T, Okabe H, Hisatomi T, et al. Ultrastructure of the trabecular meshwork in secondary glaucoma eyes after intravitreal triamcinolone acetonide. J Glaucoma. 2006; 15:117–9.

27. Jones R 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006; 17:163–7.
28. Wordinger RJ, Clark AF. Effects of glucocorticoids on the trabecular meshwork: towards a better understanding of glaucoma. Prog Retin Eye Res. 1999; 18:629–67.

29. Kersey JP, Broadway DC. Corticosteroid-induced glaucoma: a review of the literature. Eye (Lond). 2006; 20:407–16.

30. Kiddee W, Trope GE, Sheng L, et al. Intraocular pressure monitoring post intravitreal steroids: a systematic review. Surv Ophthalmol. 2013; 58:291–310.

31. Kang HK, Chin HS. Complications after intravitreal triamcinolone acetonide injection: incidence and risk factors. J Korean Ophthalmol Soc. 2012; 53:76–86.

32. Rubin B, Taglienti A, Rothman RF, et al. The effect of selective laser trabeculoplasty on intraocular pressure in patients with intravitreal steroid-induced elevated intraocular pressure. J Glaucoma. 2008; 17:287–92.

33. Lee JH, Seong MC, Cho HY, Lee YJ. The effectiveness of selective laser trabeculoplasty in steroid-induced ocular hypertension. J Korean Ophthalmol Soc. 2011; 52:876–80.

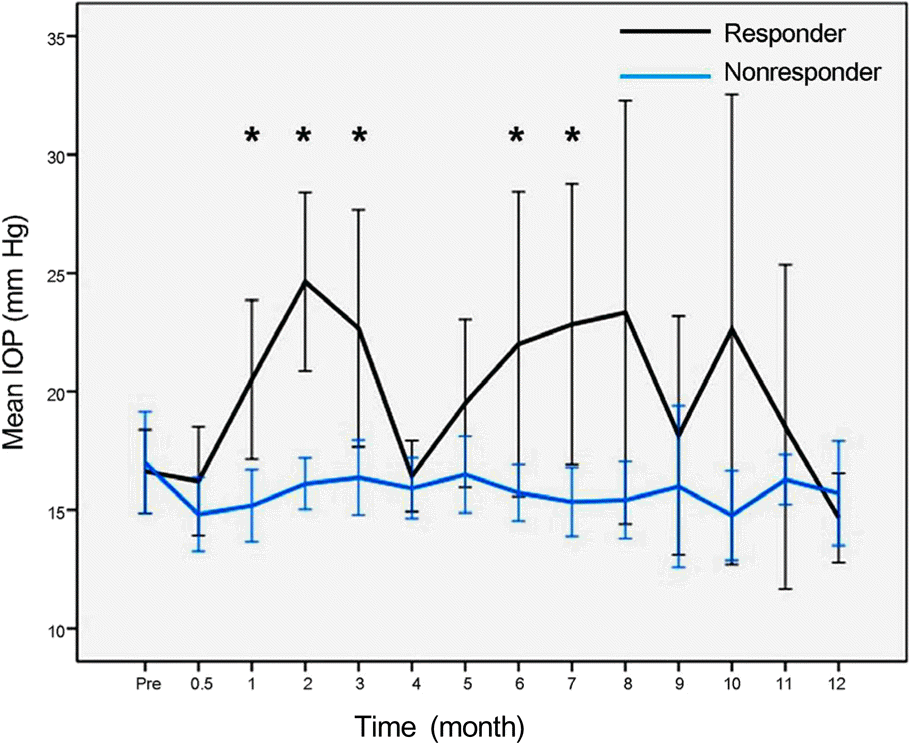
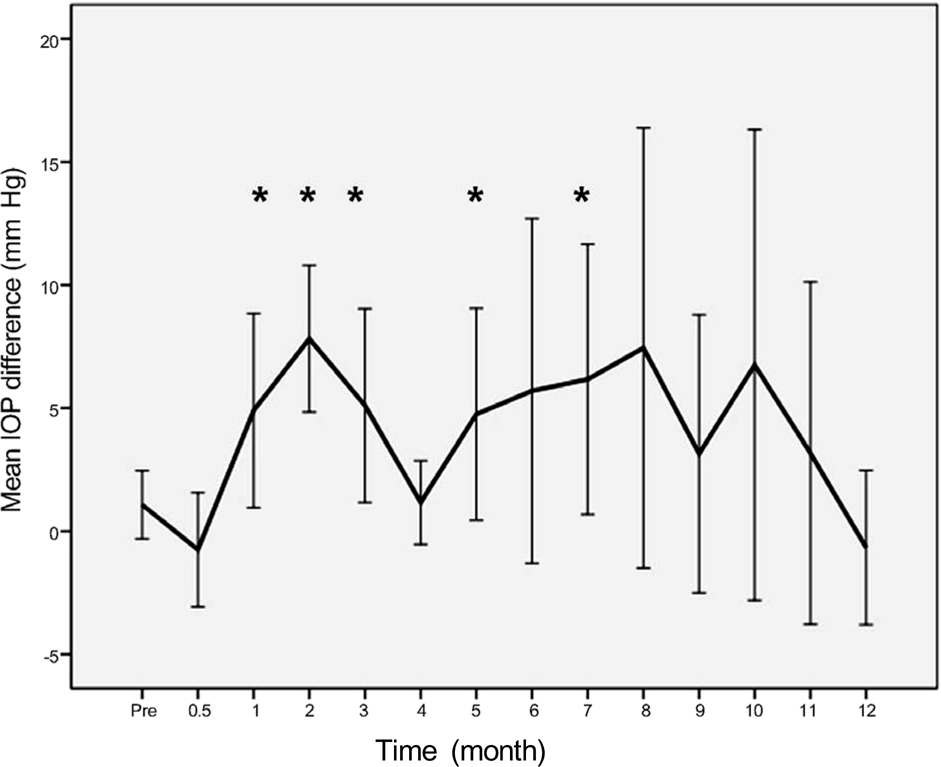
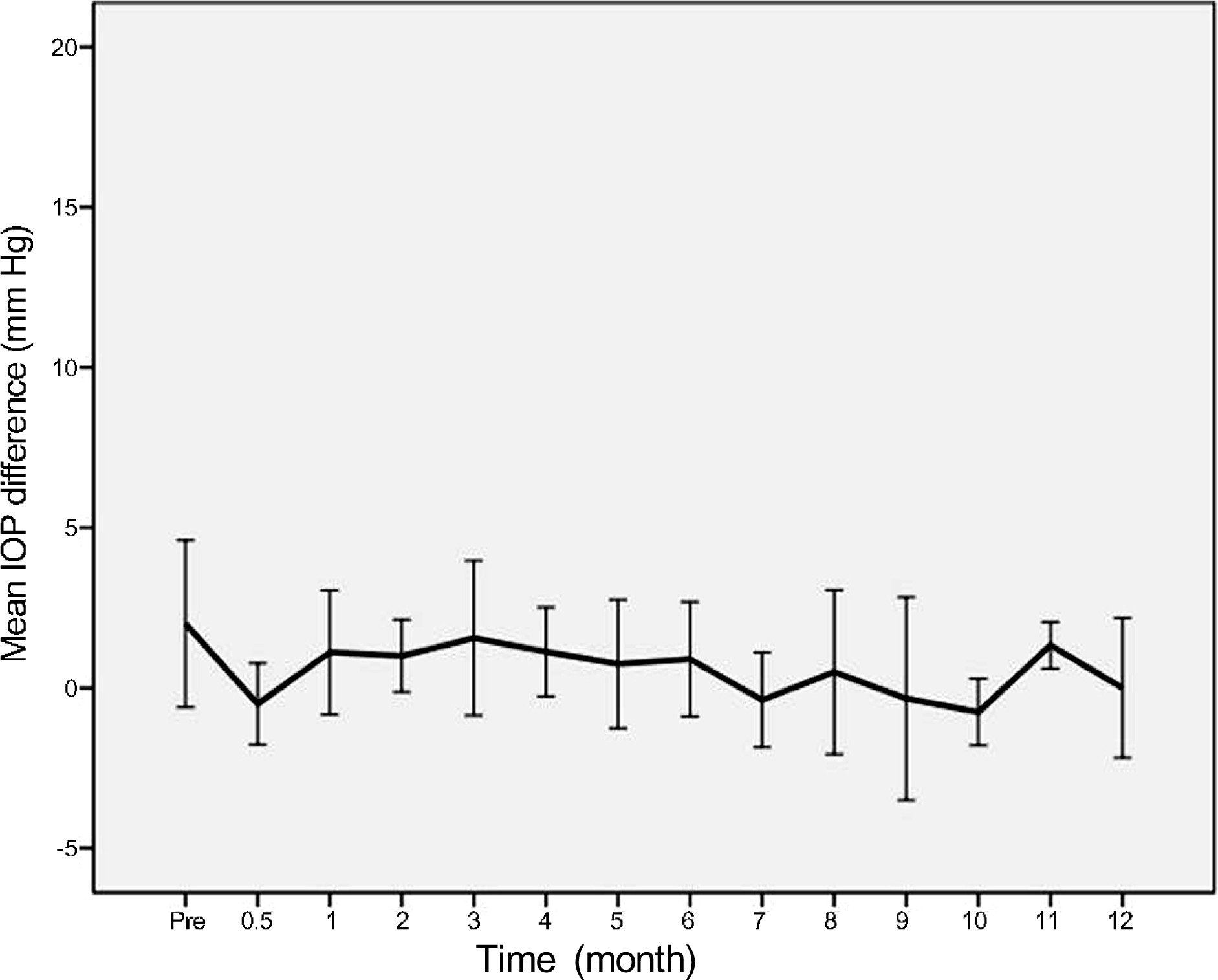
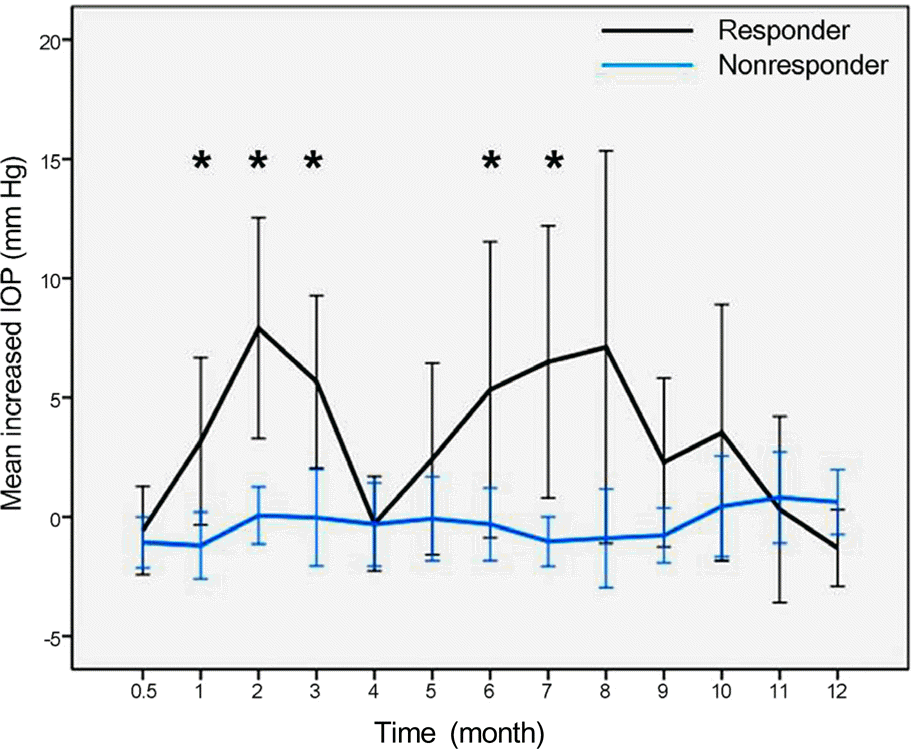




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download