Abstract
Purpose
To evaluate the effect of subconjunctival bevacizumab injection before conjunctival autograft for pterygium.
Methods
Twenty-five eyes (25 patients) with pterygium received a subconjunctival injection of 2.5 mg (0.1 mL) bevacizumab 1-2 weeks prior to conjunctival autograft surgery. The control group (25 eyes of 25 patients) received the same operation. Two weeks, 1 month and every month after the surgery, the vascularization of surgical site, the recurrence rate and the effect of wound healing were analyzed.
Results
The bevacizumab group showed a decreased conjunctival vascularity grade compared with the control group based on light microscopy. The bevacizumab group also showed lower vascular epithelial growth factor (VEGF) compared with the control group using immunohistochemical analysis and western blot. There was no recurrence in both groups, but, persistent autograft edema was observed at 8 weeks postoperatively in the bevacizumab group.
Conclusions
Although preoperative injection of bevacizumab effectively reduced vascularity and VEGF concentration of pterygium tissue, prolonged autograft edema was observed. Based on these results, bevacizumab inhibits lymphangiogenesis as well as angiogenesis. Therefore, delayed wound healing should be considered when subconjunctival bevacizumab injection is administered before pterygium surgery.
Go to : 
References
2. Wong WW. A hypothesis on the pathogenesis of pterygiums. Ann Ophthalmol. 1978; 10:303–8.
3. Coroneo MT. Pterygium as an early indicator of ultraviolet in-solation: a hypothesis. Br J Ophthalmol. 1993; 77:734–9.

4. Kria L, Ohira A, Amemiya T. Immunohistochemical localization of basic fibroblast growth factor, platelet derived growth factor, transforming growth factor-beta and tumor necrosis factor-alpha in the pterygium. Acta Histochem. 1996; 98:195–201.
5. Kria L, Ohira A, Amemiya T. Growth factors in cultured pterygium fibroblasts: immunohistochemical and ELISA analysis. Graefes Arch Clin Exp Ophthalmol. 1998; 236:702–8.

6. Di Girolamo N, Coroneo MT, Wakefield D. Active matrilysin (MMP-7) in human pterygia: potential role in angiogenesis. Invest Ophthalmol Vis Sci. 2001; 42:1963–8.
7. Lee DH, Cho HJ, Kim JT, et al. Expression of vascular endothelial growth factor and inducible nitric oxide synthase in pterygia. Cornea. 2001; 20:738–42.

8. Marcovich AL, Morad Y, Sandbank J, et al. Angiogenesis in pterygium: morphometric and immunohistochemical study. Curr Eye Res. 2002; 25:17–22.

9. Aspiotis M, Tsanou E, Gorezis S, et al. Angiogenesis in pterygium: study of microvessel density, vascular endothelial growth factor, and thrombospondin-1. Eye (Lond). 2007; 21:1095–101.

10. Gebhardt M, Mentlein R, Schaudig U, et al. Differential expression of vascular endothelial growth factor implies the limbal origin of pterygia. Ophthalmology. 2005; 112:1023–30.

11. Jin J, Guan M, Sima J, et al. Decreased pigment epithelium-derived factor and increased vascular endothelial growth factor levels in pterygia. Cornea. 2003; 22:473–7.

12. Razeghinejad MR, Hosseini H, Ahmadi F, et al. Preliminary results of subconjunctival bevacizumab in primary pterygium excision. Ophthalmic Res. 2010; 43:134–8.

13. Shenasi A, Mousavi F, Shoa-Ahari S, et al. Subconjunctival bevacizumab immediately after excision of primary pterygium: the first clinical trial. Cornea. 2011; 30:1219–22.

14. Teng CC, Patel NN, Jacobson L. Effect of subconjunctival bevacizumab on primary pterygium. Cornea. 2009; 28:468–70.

15. Banifatemi M, Razeghinejad MR, Hosseini H, Gholampour A. Bevacizumab and ocular wound healing after primary pterygium excision. J Ocul Pharmacol Ther. 2011; 27:17–21.

16. Suh JS, Choi SK. The effect of subconjunctival bevacizumab injection after primary pterygium surgery. J Korean Ophthalmol Soc. 2013; 54:53–9.

17. Lee JW, Park YJ, Kim IT, Lee KW. Clinical results after application of bevacizumab in recurrent pterygium. J Korean Ophthalmol Soc. 2008; 49:1901–9.

18. Tan DT, Chee SP, Dear KB, Lim AS. Effect of pterygium morphol-ogy on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol. 1997; 115:1235–40.

19. Kim HH, Mun HJ, Park YJ, et al. Conjunctivolimbal autograft using a fibrin adhesive in pterygium surgery. Korean J Ophthalmol. 2008; 22:147–54.

20. Ang LP, Chua JL, Tan DT. Current concepts and techniques in pterygium treatment. Curr Opin Ophthalmol. 2007; 18:308–13.

21. Todani A, Melki SA. Pterygium: current concepts in pathogenesis and treatment. Int Ophthalmol Clin. 2009; 49:21–30.
22. Rubinfeld RS, Pfister RR, Stein RM, et al. Serious complications of topical mitomycin-C after pterygium surgery. Ophthalmology. 1992; 99:1647–54.

23. Bekibele CO, Baiyeroju AM, Ajayi BG. 5-fluorouracil vs. beta-ir-radiation in the prevention of pterygium recurrence. Int J Clin Pract. 2004; 58:920–3.

24. Prabhasawat P, Tesavibul N, Leelapatranura K, Phonjan T. Efficacy of subconjunctival 5-fluorouracil and triamcinolone injection in impending recurrent pterygium. Ophthalmology. 2006; 113:1102–9.

25. Dadeya S, Kamlesh . Intraoperative daunorubicin to prevent the recurrence of pterygium after excision. Cornea. 2001; 20:172–4.

26. Di Girolamo N, Wakefield D, Coroneo MT. UVB-mediated in-duction of cytokines and growth factors in pterygium epithelial cells involves cell surface receptors and intracellular signaling. Invest Ophthalmol Vis Sci. 2006; 47:2430–7.

27. Wilgus TA, Ferreira AM, Oberyszyn TM, et al. Regulation of scar formation by vascular endothelial growth factor. Lab Invest. 2008; 88:579–90.

28. Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005; 333:328–35.

29. Lazic R, Gabric N. Intravitreally administered bevacizumab (Avastin) in minimally classic and occult choroidal neovascularization secondary to age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2007; 245:68–73.

30. Jorge R, Costa RA, Calucci D, et al. Intravitreal bevacizumab (Avastin) for persistent new vessels in diabetic retinopathy (IBEPE study). Retina. 2006; 26:1006–13.

31. Iliev ME, Domig D, Wolf-Schnurrbursch U, et al. Intravitreal bevacizumab (Avastin) in the treatment of neovascular glaucoma. Am J Ophthalmol. 2006; 142:1054–6.

32. Jeong JH, Chun YS, Kim JC. The effects of a subtenoncapsular injection of bevacizumab for ocular surface disease with corneal neovascularization. J Korean Ophthalmol Soc. 2009; 50:1475–82.

33. Manzano RP, Peyman GA, Khan P, et al. Inhibition of experimental corneal neovascularisation by bevacizumab (Avastin). Br J Ophthalmol. 2007; 91:804–7.

34. DeStafeno JJ, Kim T. Topical bevacizumab therapy for corneal neovascularization. Arch Ophthalmol. 2007; 125:834–6.

35. Bock F, Onderka J, Dietrich T, et al. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest Ophthalmol Vis Sci. 2007; 48:2545–52.

36. Hosseini H, Nejabat M. A potential therapeutic strategy for inhibition of corneal neovascularization with new anti-VEGF agents. Med Hypotheses. 2007; 68:799–801.

37. Enkvetchakul O, Thanathanee O, Rangsin R, et al. A randomized controlled trial of intralesional bevacizumab injection on primary pterygium: preliminary results. Cornea. 2011; 30:1213–8.

Go to : 
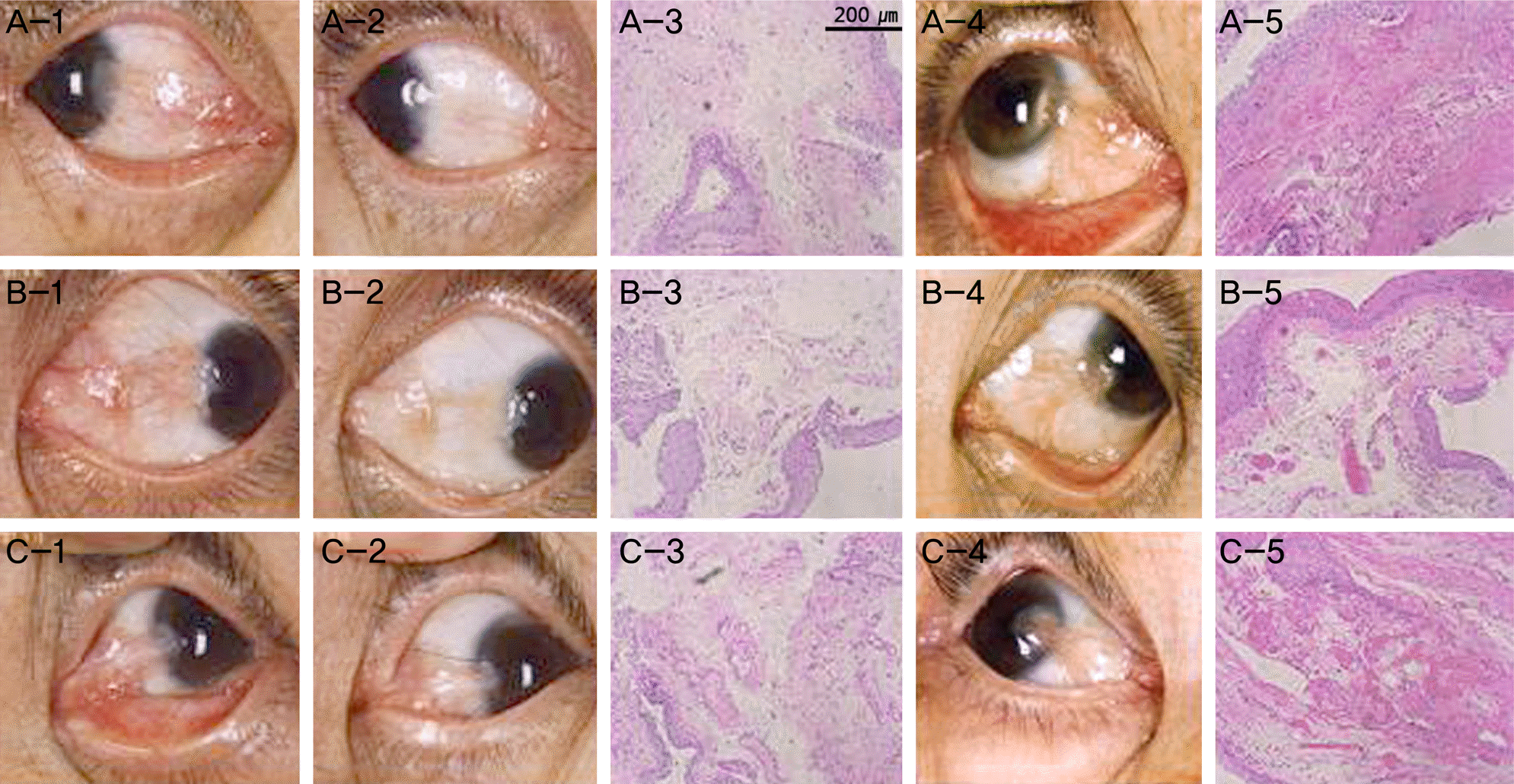 | Figure 1.Comparison vascularization of pterygium tissue obtained from pterygium surgery in cases treated with bevacizumab, and the control group. (A-1, B-1) Anterior segment photography of primary pterygium cases before injection of bevacizumab. (C-1) Anterior segment photography of recurrent pterygium cases before injection of bevacizumab. (A-2, B-2, C-2) Anterior segment photography shows decreased conjuntival injection after injection of bevacizumab, but before the surgery. (A-3, B-3, C-3) LM (H&E stain, ×100) of biopsy obtained from pterygium surgery. (A-4, B-4, C-4) Control group of similar degree of pterygium without bevacizumab injection. (A-4, B-4) primary pterygium (C-4) recurrent pterygium. (A-5, B-5, C-5) LM of biopsy form the above-mentioned (A-4, B-4, C-4) control group. These results show marked decrease in vascularization grade in group with bevacizumab treatment compared to the control group. LM = light microscopy (Axio Vision 4; Carl Zeiss, Jena, Germany). |
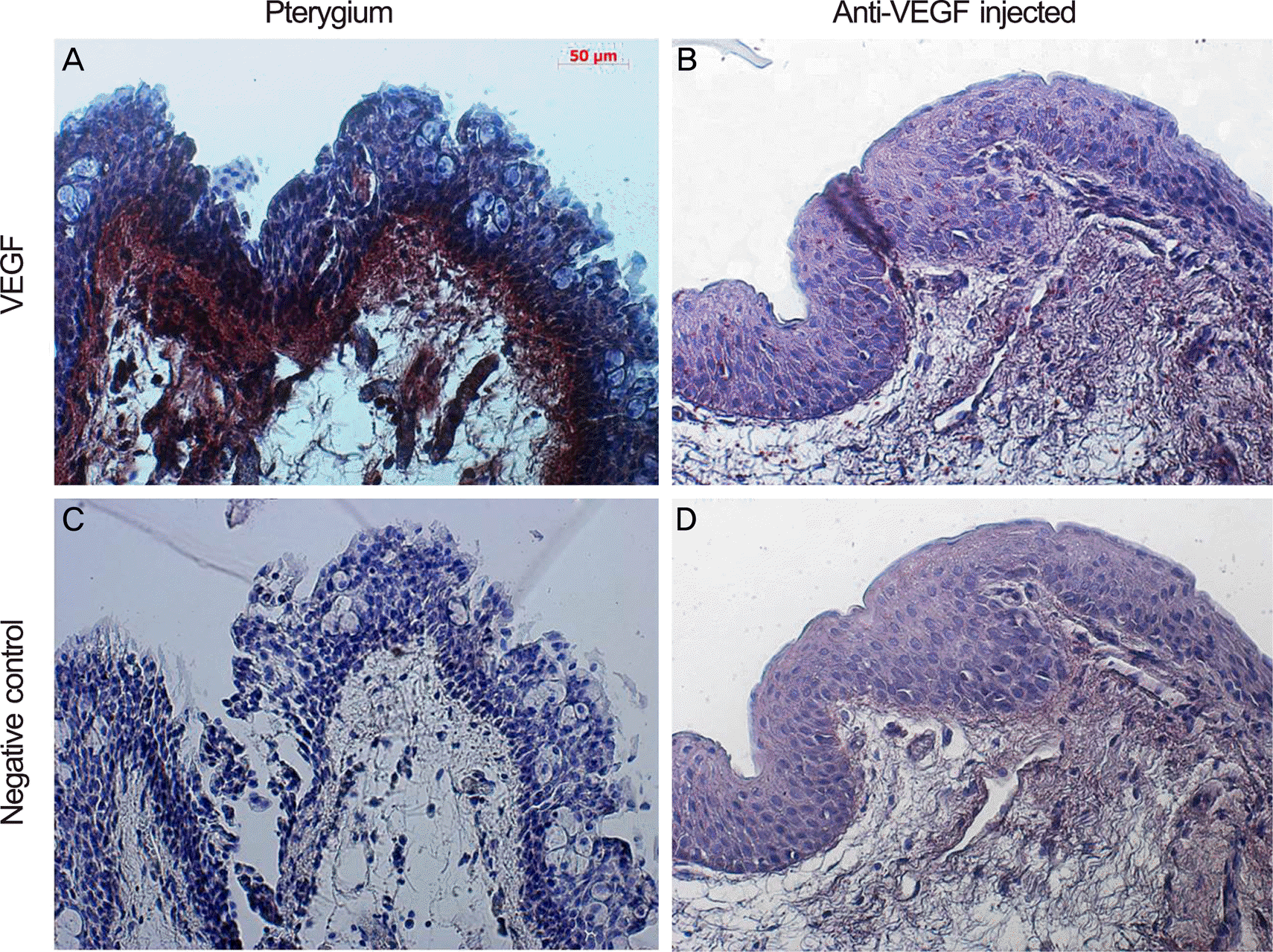 | Figure 2.LM immunohistochemistry for VEGF (peroxidase stain, ×100) of pterygium tissue form pterygium surgery in control group (A, C) and bevacizumab group (B, D). (A) and (C) show stronger stain at the apical epithelium, indicating denser concentration of VEGF than (B) and (D). VEFG=vascular endothelial growth factor; LM = light microscopy (Axio Vision 4; Carl Zeiss, Jena, Germany). |
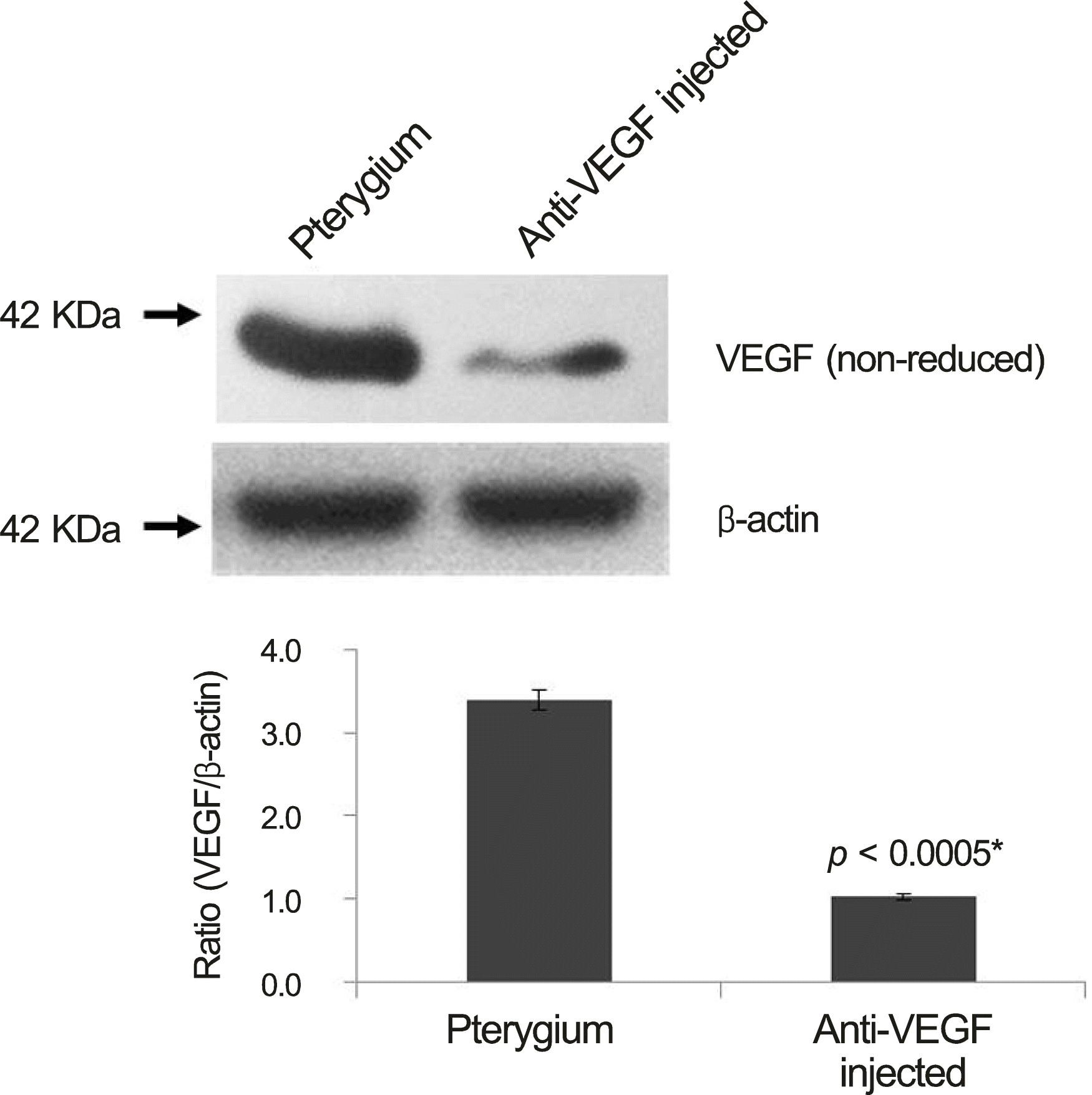 | Figure 3.Western blot for vascular endothelial growth factor (VEGF) of pterygium tissue form pterygium surgery in bevacizumab group and control group. Bevacizumab group were lower VEGF expression than control group. * Student t-test (p <0.05). |
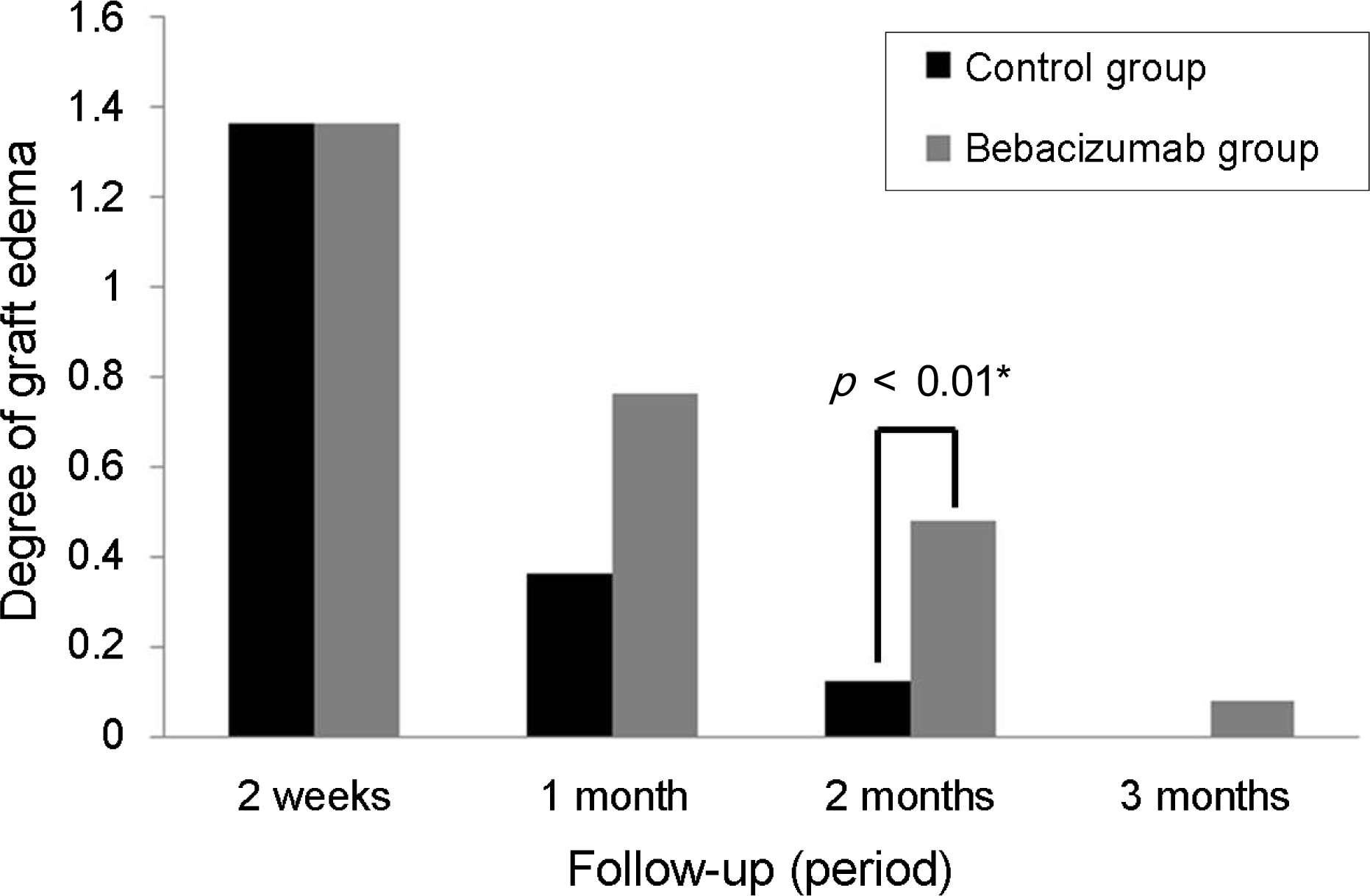 | Figure 4.Comparison of the graft edema presented at conjuntivolimbal autograft between the bevacizumab group and the control group at each follow-up. The bevacizumab group exhibits more persistent, and significant graft edema on second month follow-up after surgery. The scoring system of graft edema (0 means no edema, equal in thickness to normal conjunctiva; 1 means mild edema, graft edema within x1 of the corneal thickness under slit-lamp exam; 2 means moderate edema, between 1-3; 3 means severe edema, graft edema above x2 of the corneal thickness under slit-lamp exam). * Fisher’s exact test, p<0.05. |
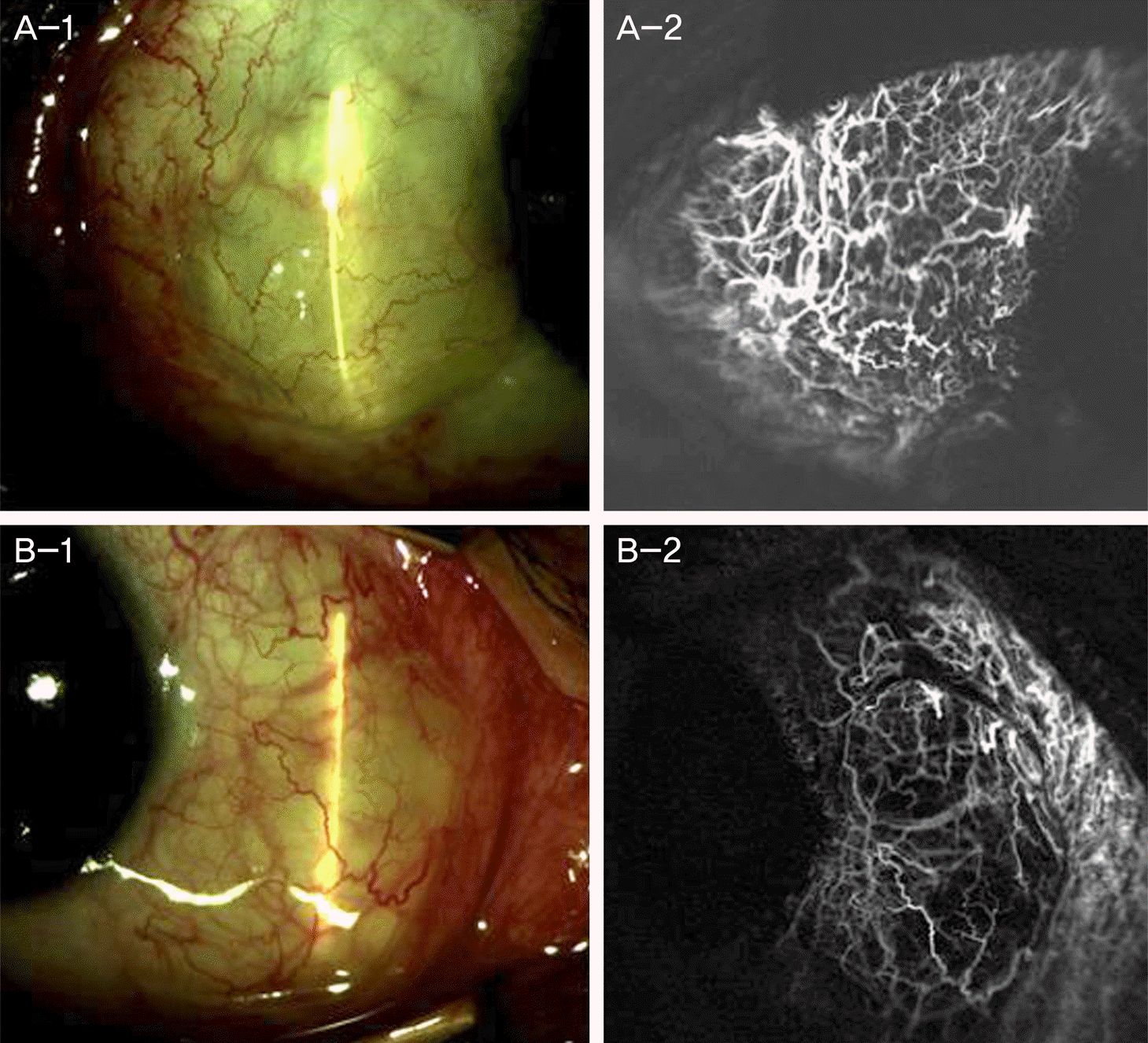 | Figure 5.Persistent post-surgical graft edema due to application of subconjuntival bevacizumab injection 2 weeks before the surgery. (A-1, B-1) Shows severe graft edema 2 months after surgery. (A-2, B-2) Shows normal connection between graft vessels and recipient vessels. |
Table 1.
Demography of Bevacizumab group* and control group
|
Bevacizumab group* |
Control group |
|||
|---|---|---|---|---|
| Rucurrent pterygium | Primary pterygium | Recurrent pterygium | Primary pterygium | |
| No. of eyes | 7 | 18 | 7 | 18 |
| M:F | 2:5 | 11:7 | 2:5 | 11:7 |
| Age (years) | 52.43 ± 8.18 | 53.72 ± 10.80 | 53.71 ± 4.99 | 54.38 ± 9.89 |
| Follow up (months) | 14.86 ± 3.80 | 13.44 ± 2.36 | 13.86 ± 2.04 | 13.67 ± 2.59 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download