초록
Purpose:
To investigate the effects of nitric oxide (NO) on the permeability of cultured human trabecular meshwork cell (HTMC) monolayer.
Methods:
HTMCs were cultured until confluency in the Transwell inner chamber and then exposed to 0, 10 or 100 μ m S-nitro-so-N-acetyl-DL-penicillamine (SNAP) and 0.5 mm L-N G-Nitroarginine methyl ester (L-NAME) for 24 hours. Permeabilities of carboxyfluorescein through the HTMC monolayer were measured using a spectrofluorometer after 2 hours in the outer chamber. Cellular viabilities and production of NO were assessed using 3-(4, 5 – dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) and Griess assay, respectively.
Results:
The cellular survival was not affected by 10 or 100 μ m SNAP ( p > 0.05) but NO production increased in a dose-depend-ent manner ( p < 0.05). SNAP significantly increased the permeability of carboxyfluorescein through the HTMC monolayer in a dose-dependent manner compared with non-exposed control ( p < 0.05). The endothelial NO synthase inhibitor L-NAME abolished SNAP-induced increase of the carboxyfluorescein permeability ( p > 0.05).
Go to : 
References
1. Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984; 91:564–79.

2. Rohen JW, Lütjen-Drecoll E, Flügel C, et al. Ultrastructure of the trabecular meshwork in untreated cases of primary open-angle glaucoma (POAG). Exp Eye Res. 1993; 56:683–92.
3. Nathanson JA, McKee M. Identification of an extensive system of nitric oxide-producing cells in the ciliary muscle and outflow path-way of the human eye. Invest Ophthalmol Vis Sci. 1995; 36:1765–73.
4. Geyer O, Podos SM, Mittag T. Nitric oxide synthase activity in tissues of the bovine eye. Graefes Arch Clin Exp Ophthalmol. 1997; 235:786–93.

5. Meyer P, Champion C, Schlötzer-Schrehardt U, et al. Localization of nitric oxide synthase isoforms in porcine ocular tissues. Curr Eye Res. 1999; 18:375–80.

6. Wiederholt M, Sturm A, Lepple-Wienhues A. Relaxation of trabecular meshwork and ciliary muscle by release of nitric oxide. Invest Ophthalmol Vis Sci. 1994; 35:2515–20.
7. Behar-Cohen FF, Goureau O, D'Hermies F, Courtois Y. Decreased intraocular pressure induced by nitric oxide donors is correlated to nitrite production in the rabbit eye. Invest Ophthalmol Vis Sci. 1996; 37:1711–5.
8. Nathanson JA, McKee M. Alterations of ocular nitric oxide synthase in human glaucoma. Invest Ophthalmol Vis Sci. 1995; 36:1774–84.
9. Araie M. Carboxyfluorescein. A dye for evaluating the corneal endothelial barrier function in vivo. Exp Eye Res. 1986; 42:141–50.

10. Araie M. Barrier function of corneal endothelium and the intraocular irrigating solutions. Arch Ophthalmol. 1986; 104:435–8.

11. Tsuboi S, Pederson JE. Permeability of the isolated dog retinal pigment epithelium to carboxyfluorescein. Invest Ophthalmol Vis Sci. 1986; 27:1767–70.
12. Blair NP, Rusin MM. Blood-retinal barrier permeability to carboxyfluorescein and fluorescein in monkeys. Graefes Arch Clin Exp Ophthalmol. 1986; 224:419–22.

13. Grimes PA. Carboxyfluorescein transfer across the blood-retinal barrier evaluated by quantitative fluorescence microscopy: comparison with fluorescein. Exp Eye Res. 1988; 46:769–83.

14. Nakagawa S, Usui T, Yokoo S, et al. Toxicity evaluation of anti-glaucoma drugs using stratified human cultivated corneal epithelial sheets. Invest Ophthalmol Vis Sci. 2012; 53:5154–60.

15. Kameda T, Inoue T, Inatani M, et al. The effect of Rho-associated protein kinase inhibitor on monkey Schlemm's canal endothelial cells. Invest Ophthalmol Vis Sci. 2012; 53:3092–103.

16. Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001; 42:1029–37.
17. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.

18. Freimoser FM, Jakob CA, Aebi M, Tuor U. The MTT [3-(4,5-di-methylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay is a fast and reliable method for colorimetric determination of fungal cell densities. Appl Environ Microbiol. 1999; 65:3727–9.

19. Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982; 126:131–8.

20. Stamer WD, Lei Y, Boussommier-Calleja A, et al. eNOS, a pressure-dependent regulator of intraocular pressure. Invest Ophthalmol Vis Sci. 2011; 52:9438–44.

21. Predescu D, Predescu S, Shimizu J, et al. Constitutive eNOS-de-rived nitric oxide is a determinant of endothelial junctional integrity. Am J Physiol Lung Cell Mol Physiol. 2005; 289:L371–81.

Go to : 
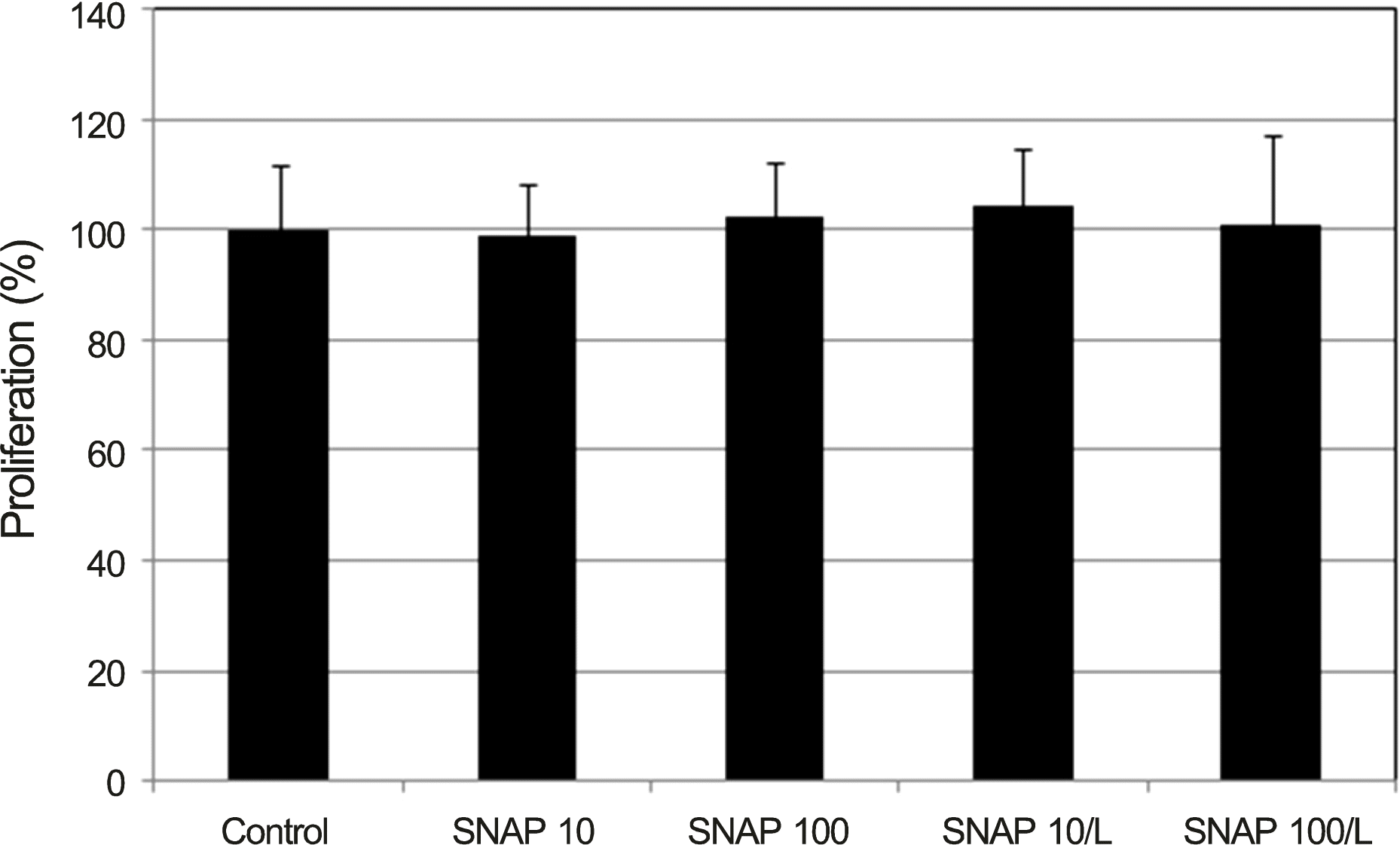 | Figure 1.Effect of nitric oxide donor on the survival of confluently cultured trabecular meshwork cells in monolayer. 10, 100 μ m SNAP and co-exposed 0.5 mm L did not affect on the survival significantly compared to non-exposed control ( p > 0.05). SNAP = S-Nitroso-N-acetyl-DL-penicillamine; L = L-N G- Nitroarginine methyl ester. |
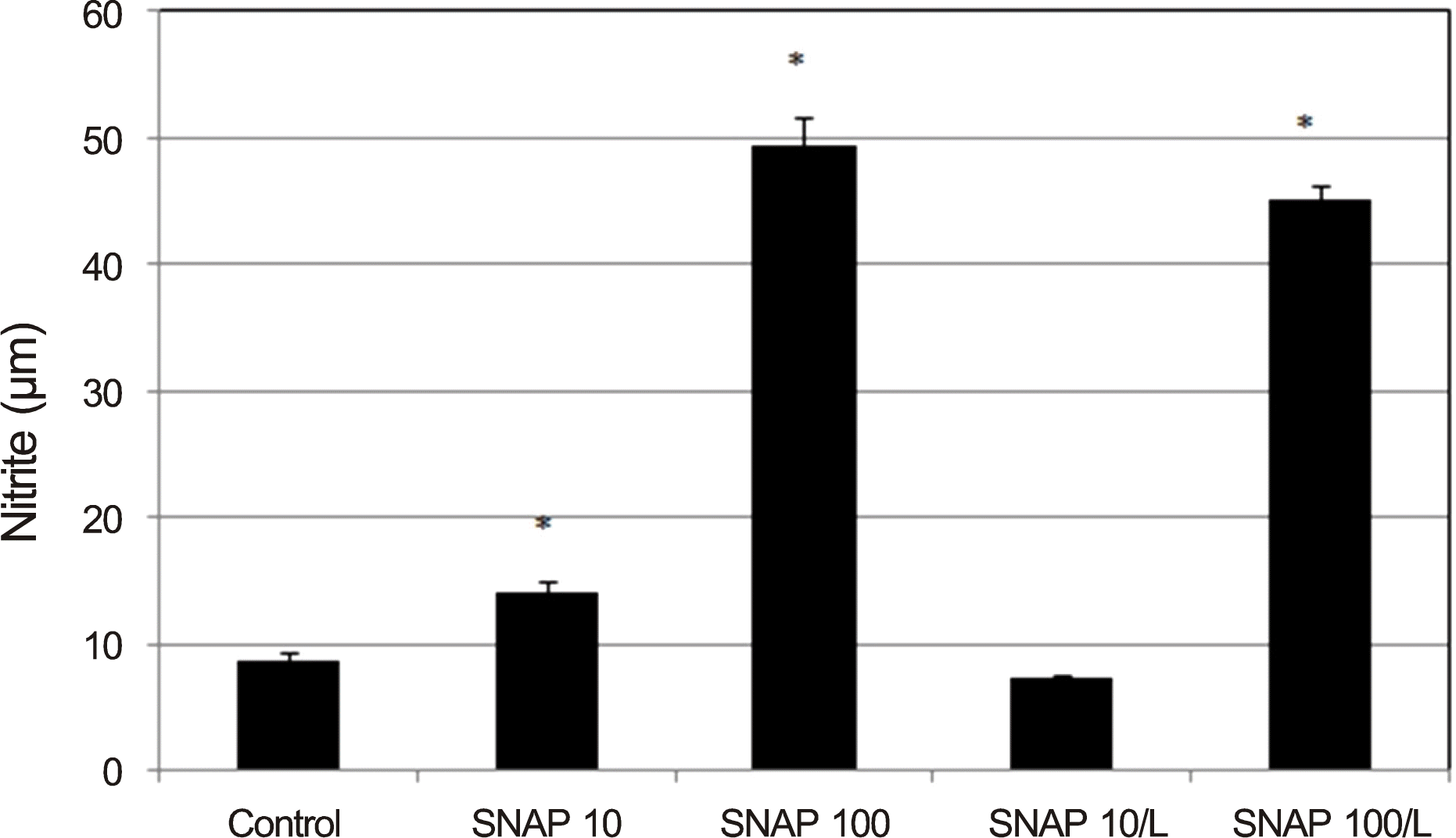 | Figure 2.Effect of nitric oxide donor on the production of nitrite in confluently cultured trabecular meshwork cells. 10, 100 μ m SNAP, and 100 μ m SNAP with 0.5 mm L increased nitrite production significantly compared to non-exposed control. SNAP = S-Nitroso-N-acetyl-DL-penicillamine; L = L-N G-Nitroarginine methyl ester. * p < 0.05. |
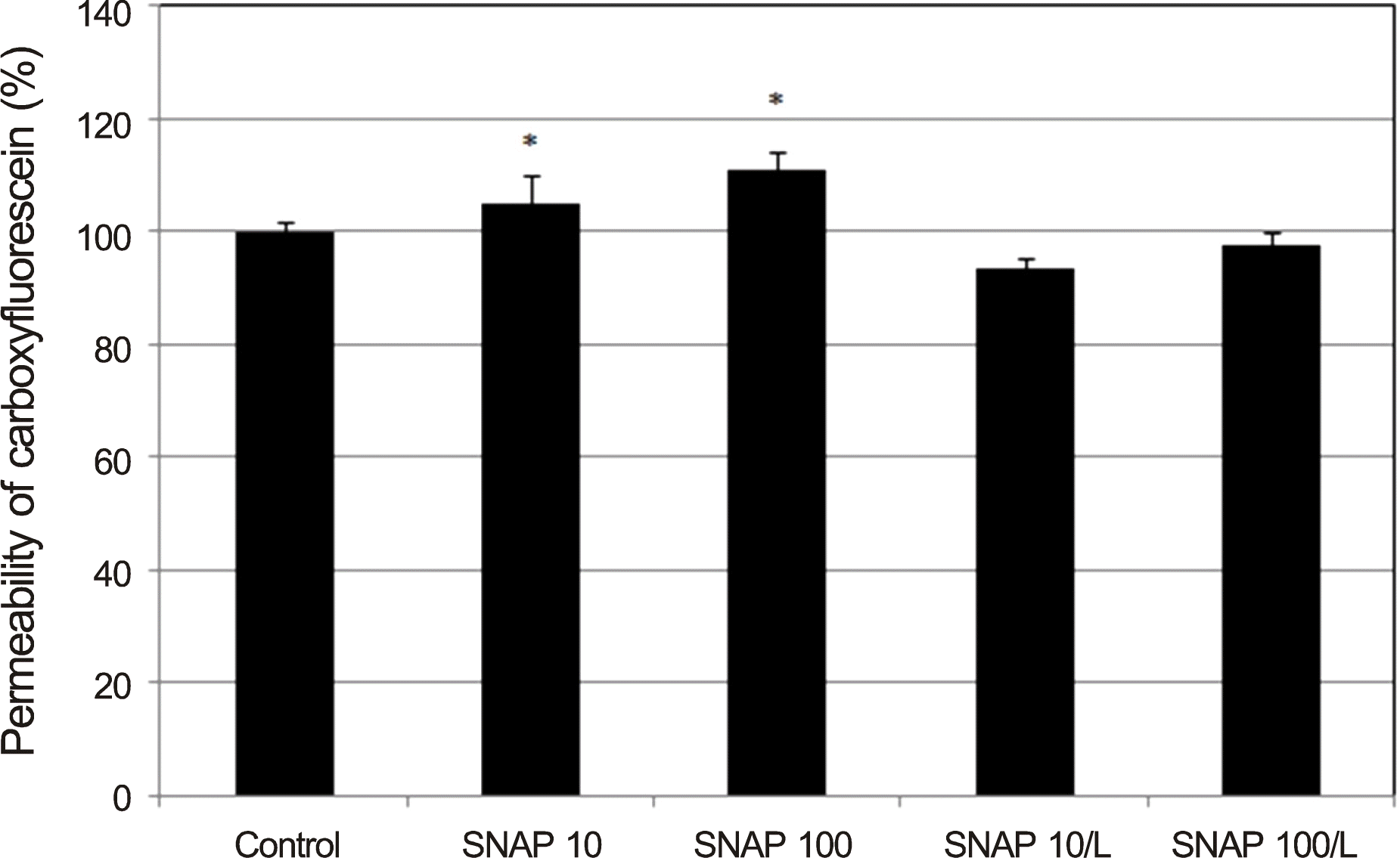 | Figure 3.Effects of nitric oxide donor on the permeability of carboxyfluorescin through the trabecular meshwork cell monolayer. 10, 100 μ m SNAP increased the permeability of carboxyfluorescin significantly compared to non-exposed control and abolished by co-exposed 0.5 mm L. Carboxyfluorescein intensity of outer chamber normalized to the mean value obtained using non-ex-posed control (permeability 100%). SNAP = S-Nitroso-N- ace-tyl-DL-penicillamine; L = L-N G-Nitroarginine methyl ester. * p < 0.05. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download