초록
Purpose:
Non-intraocular pressure (IOP) factors such as vascular factors have been identified as contributing to normal tension glaucoma. However, there is not an established range of haemorheological factors considered normal, nor are there stand-ardized tests. In this study, we investigated differences in blood viscosity and haemorheological parameters between patients with normal tension glaucoma (NTG) and normal controls using a new instrument called the BVD-RO1 (BIO-VISCO. Inc., Jeonju, Korea).
Methods:
Twenty patients with NTG and 20 age-matched normal controls were included in the study. Haemorheological parameters of the venous blood samples, including blood viscosity at the shear rates of 300 (high shear rate) and 1 (low shear rate) s−1 were measured using an automated scanning capillary tube viscometer.
Go to : 
References
1. Silver DM, Farrell RA, Langham ME, et al. Estimation of pulsatile ocular blood flow from intraocular pressure. Acta Ophthalmol Suppl. 1989; 191:25–9.

2. Hamard P, Hamard H, Dufaux J, Quesnot S. Optic nerve head blood flow using a laser Doppler velocimeter and haemorheology in primary open angle glaucoma and normal pressure glaucoma. Br J Ophthalmol. 1994; 78:449–53.

3. Fontana L, Poinoosawmy D, Bunce CV, et al. Pulsatile ocular blood flow investigation in asymmetric normal tension glaucoma and normal subjects. Br J Ophthalmol. 1998; 82:731–6.

4. Flammer J, Orgül S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002; 21:359–93.

5. Lowe GD. Fibrinogen and cardiovascular disease: historical introduction. Eur Heart J. 1995; 16(Suppl A):2–5.

6. Lowe GD, Lee AJ, Rumley A, et al. Blood viscosity and risk of cardiovascular events: the Edinburgh Artery Study. Br J Haematol. 1997; 96:168–73.

7. Rampling MW. Hyperviscosity as a complication in a variety of disorders. Semin Thromb Hemost. 2003; 29:459–65.

8. Stoltz JF. Hemorheology in Practice. 1st ed.Amsterdam: IOS press;1999.
9. Alexy T, Wenby RB, Pais E, et al. An automated tube-type blood viscometer: validation studies. Biorheology. 2005; 42:237–47.
10. Kim S, Cho YI, Hogenauer WN, Kensey KR. A method of isolating surface tension and yield stress effects in a U-shaped scanning capillary-tube viscometer using a Casson model. Journal of Non-Newtonian Fluid Mechanics. 2002; 103:205–19.

11. Chung TW, Ho CP. Changes in viscosity of low shear rates and viscoelastic properties of oxidative erythrocyte suspensions. Clin Hemorheol Microcirc. 1999; 21:99–103.
12. Flammer J, Orgül S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002; 21:359–93.

13. O’Brien C, Butt Z, Ludlam C, Detkova P. Activation of the coagu-lation cascade in untreated primary open-angle glaucoma. Ophthalmology. 1997; 104:725–9. discussion 729-30.

14. Cheng HC, Chan CM, Yeh SI, et al. The hemorheological mechanisms in normal tension glaucoma. Curr Eye Res. 2011; 36:647–53.

15. Klaver JH, Greve EL, Goslinga H, et al. Blood and plasma viscosity measurements in patients with glaucoma. Br J Ophthalmol. 1985; 69:765–70.

16. Trope GE, Salinas RG, Glynn M. Blood viscosity in primary open-angle glaucoma. Can J Ophthalmol. 1987; 22:202–4.
17. Danesh J, Collins R, Peto R, Lowe GD. Haematocrit, viscosity, erythrocyte sedimentation rate: meta-analyses of prospective studies of coronary heart disease. Eur Heart J. 2000; 21:515–20.

18. Damaske A, Muxel S, Fasola F, et al. Peripheral hemorheological and vascular correlates of coronary blood flow. Clin Hemorheol Microcirc. 2011; 49:261–9.

19. Olausson EA, Kilander A. Glycaemic index of modified cornstarch in solutions with different viscosity. A study in subjects with diabetes mellitus type 2. Clin Nutr. 2008; 27:254–7.

20. Tikhomirova IA, Oslyakova AO, Mikhailova SG. Microcirculation and blood rheology in patients with cerebrovascular disorders. Clin Hemorheol Microcirc. 2011; 49:295–305.

21. Vayá A, Hernández-Mijares A, Bonet E, et al. Association between hemorheological alterations and metabolic syndrome. Clin Hemorheol Microcirc. 2011; 49:493–503.

22. Rillaerts E, van Gaal L, Xiang DZ, et al. Blood viscosity in human obesity: relation to glucose tolerance and insulin status. Int J Obes. 1989; 13:739–45.
23. Li RY, Cao ZG, Li Y, Wang RT. Increased whole blood viscosity is associated with silent cerebral infarction. Clin Hemorheol Microcirc. 2013 Aug 29; [Epub ahead of print].

24. Ehrly AM. Therapeutic hemorheology. 1st ed.New York: Springer-Verlag;1991.
25. Koscielny J, Kiesewetter H, Jung F, Haaβ A. Hemodilution. 1st ed.Berlin: Springer-Verlag;1992.
26. Cokelet GR. The rheology and tube flow of blood. Skalak R, Chien S, editors. Handbook of bioengineering. 1st ed.New York: McGraw-Hill;1987. p. 1–17.
27. Rosenson RS. Viscosity, and ischemic heart disease. J Vasc Med Biol. 1993; 4:206–12.
28. Cho YI, Kim WT, Kensey KR. A new scanning capillary tube viscometer. Rev Sci Instrum. 1999; 70:2421–3.

29. Késmárky G, Kenyeres P, Rábai M, Tóth K. Plasma viscosity: a forgotten variable. Clin Hemorheol Microcirc. 2008; 39:243–6.

30. Cecchi E, Giglioli C, Valente S, et al. Role of hemodynamic shear stress in cardiovascular disease. Atherosclerosis. 2011; 214:249–56.

31. Neumann FJ, Katus HA, Hoberg E, et al. Increased plasma viscosity and erythrocyte aggregation: indicators of an unfavourable clinical outcome in patients with unstable angina pectoris. Br Heart J. 1991; 66:425–30.

32. Baskurt OK, Meiselman HJ. Blood rheology and hemodynamics. Semin Thromb Hemost. 2003; 29:435–50.

33. de Simone G, Devereux RB, Chien S, et al. Relation of blood viscosity to demographic and physiologic variables and to cardiovascular risk factors in apparently normal adults. Circulation. 1990; 81:107–17.

34. Ditzel J, Kampmann J. Whole-blood viscosity, hematocrit and plasma protein in normal subjects at different ages. Acta Physiol Scand. 1971; 81:264–8.
35. Rosenson RS, McCormick A, Uretz EF. Distribution of blood viscosity values and biochemical correlates in healthy adults. Clin Chem. 1996; 42(8 Pt 1):1189–95.

36. Mayer GA. Blood viscosity in healthy subjects and patients with coronary heart disease. Can Med Assoc J. 1964; 91:951–4.
37. Letcher RL, Chien S, Pickering TG, et al. Direct relationship between blood pressure and blood viscosity in normal and hyper-tensive subjects. Role of fibrinogen and concentration. Am J Med. 1981; 70:1195–202.
38. Litwin MS, Chapman K, Stoliar JB. Blood viscosity in the normal man. Surgery. 1970; 67:342–5.
39. Rand PW, Lacombe E, Hunt HE, Austin WH. Viscosity of normal human blood under normothermic and hypothermic conditions. J Appl Physiol. 1964; 19:117–22.

40. Hall JE. Guyton and Hall textbook of medical physiology. 12th ed.Philadelphia: WB Saunders;2010.
Go to : 
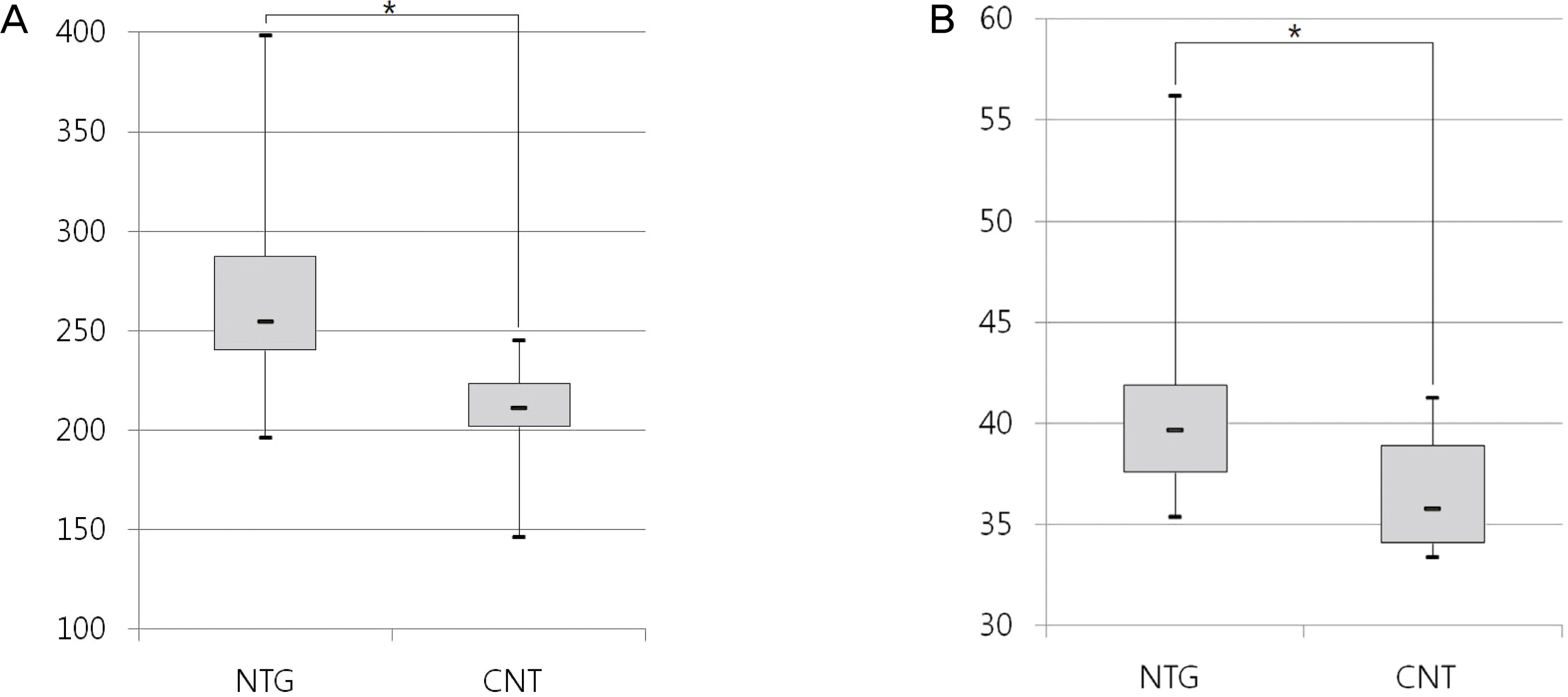 | Figure 1.Whole blood viscosity at shear rate of 1 s-1 and 300 s-1. (A) Low-shear blood viscosity in NTG and control group. (B) High-shear blood viscosity in NTG and control group. NTG = normal tension glaucoma; CNT = Control. * p < 0.05 statistically significant. |
Table 1.
Baseline clinical characterisctics and recent medication
| Normal tension glaucoma | Control | p-value* | |
|---|---|---|---|
| Age (years) | 61.2 ± 10.39 | 58.3 ± 7.86 | 0.33 |
| Male (n, %) | 9 (45) ± 0.51 | 9 (45) ± 0.51 | 1.00 |
| Height (cm) | 163.7 ± 8.25 | 163.4 ± 8.48 | 0.93 |
| Weight (kg) | 63.3 ± 8.55 | 66.7 ± 12.65 | 0.33 |
| Hypertension (n, %) | 7 (35) ± 0.49 | 8 (40) ± 0.50 | 0.75 |
| Diabetes (n, %) | 4 (20) ± 0.41 | 4 (20) ± 0.41 | 1.00 |
| Dyslipidemia (n, %) | 3 (15) ± 0.37 | 4 (20) ± 0.41 | 0.69 |
| Smoking (n, %) | 5 (25) ± 0.44 | 5 (25) ± 0.44 | 1.00 |
Table 2.
Baseline laboratory findings of normal tension glaucoma group and control group
| Normal tension glaucoma | Control | p-value* | |
|---|---|---|---|
| WBC (×103/mm3) | 7.2 ± 1.43 | 7.8 ± 0.84 | 0.08 |
| Hematocrit (%) | 42.8 ± 3.75 | 40.2 ± 3.54 | 0.04† |
| Hemoglobin (g/dL) | 14.7 ± 1.10 | 14.1 ± 1.04 | 0.10 |
| HbA1c (%) | 6.0 ± 1.40 | 6.2 ± 1.06 | 0.55 |
| Platelet (×103/mm3) | 224.7 ± 54.36 | 216 ± 25.46 | 0.52 |
| Triglyceride (mg/dL) | 127.4 ± 76.41 | 111.3 ± 34.62 | 0.40 |
| Total cholesterol (mg/dL) | 171.5 ± 25.21 | 167.8 ± 31.22 | 0.69 |
| LDL cholesterol (mg/dL) | 94.3 ± 22.47 | 92.7 ± 20.10 | 0.81 |
| HDL cholesterol (mg/dL) | 43 ±13.80 | 41.8 ± 10.32 | 0.76 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download