초록
Purpose:
In this study we determined the correlation of axial length to lamina cribrosa thickness (LCT), prelaminar tissue thickness (PT), and anterior laminar displacement (ALD) in young healthy eyes.
Methods:
The optic discs of 60 eyes from 30 young healthy subjects with myopia were scanned using enhanced-depth imaging spectral-domain optical coherence tomography (Spectralis OCT, Heidelberg Engineering, Heidelberg, Germany). The LCT, PT, and ALD were measured at the superior midperipheral, middle, and inferior midperipheral of the optic nerve head, respectively. A linear mixed-effects model was used to determine the relationship between the axial length and the LCT, axial length and PT as well as axial length and ALD.
Results:
The mean, superior midperipheral, and middle LCT were not significantly correlated with axial length. Conversely, the inferior midperipheral LCT was negatively correlated with axial length ( p = 0.019, β = -7.34). There was no significant association between axial length and PT. Mean ALD was negatively correlated with axial length ( p = 0.022, β = -17.17).
Go to : 
References
1. Xu L, Wang Y, Wang S, et al. High myopia and glaucoma susceptibility the Beijing Eye Study. Ophthalmology. 2007; 114:216–20.
2. Kuzin AA, Varma R, Reddy HS, et al. Ocular biometry and open-angle glaucoma: the Los Angeles Latino Eye Study. Ophthalmology. 2010; 117:1713–9.

3. Perera SA, Wong TY, Tay WT, et al. Refractive error, axial dimensions, and primary open-angle glaucoma: the Singapore Malay Eye Study. Arch Ophthalmol. 2010; 128:900–5.
6. Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol. 1981; 99:635–49.
7. Radius RL, Anderson DR. Rapid axonal transport in primate optic nerve. Distribution of pressure-induced interruption. Arch Ophthalmol. 1981; 99:650–4.
8. Jonas JB, Berenshtein E, Holbach L. Lamina cribrosa thickness and spatial relationships between intraocular space and cerebrospinal fluid space in highly myopic eyes. Invest Ophthalmol Vis Sci. 2004; 45:2660–5.

9. Ren R, Wang N, Li B, et al. Lamina cribrosa and peripapillary sclera histomorphometry in normal and advanced glaucomatous Chinese eyes with various axial length. Invest Ophthalmol Vis Sci. 2009; 50:2175–84.

10. Lee EJ, Kim TW, Weinreb RN, et al. Visualization of the lamina cribrosa using enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2011; 152:87–95.e1.

11. Park HY, Park CK. Diagnostic capability of lamina cribrosa thickness by enhanced depth imaging and factors affecting thickness in patients with glaucoma. Ophthalmology. 2013; 120:745–52.

12. Lee EJ, Kim TW, Weinreb RN, et al. Lamina cribrosa thickness is not correlated with central corneal thickness or axial length in healthy eyes: central corneal thickness, axial length, and lamina cribrosa thickness. Graefes Arch Clin Exp Ophthalmol. 2013; 251:847–54.
13. Lee EJ, Kim TW, Weinreb RN, et al. Visualization of the lamina cribrosa using enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2011; 152:87–95.e1.

14. McCulloch CE, Neuhaus JM. Encyclopedia of biostatistics. 2nd ed.Chichester: John Wiley and Sons;2005.
15. Reis AS, O'Leary N, Stanfield MJ, et al. Laminar displacement and prelaminar tissue thickness change after glaucoma surgery imaged with optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53:5819–26.

16. Lee EJ, Kim TW, Weinreb RN, Kim H. Reversal of lamina cribrosa displacement after intraocular pressure reduction in open-angle glaucoma. Ophthalmology. 2013; 120:553–9.

17. Lee EJ, Kim TW, Weinreb RN. Reversal of lamina cribrosa displacement and thickness after trabeculectomy in glaucoma. Ophthalmology. 2012; 119:1359–66.

18. Trible JR, Sergott RC, Spaeth GL, et al. Trabeculectomy is associated with retrobulbar hemodynamic changes. A color Doppler analysis. Ophthalmology. 1994; 101:340–51.
Go to : 
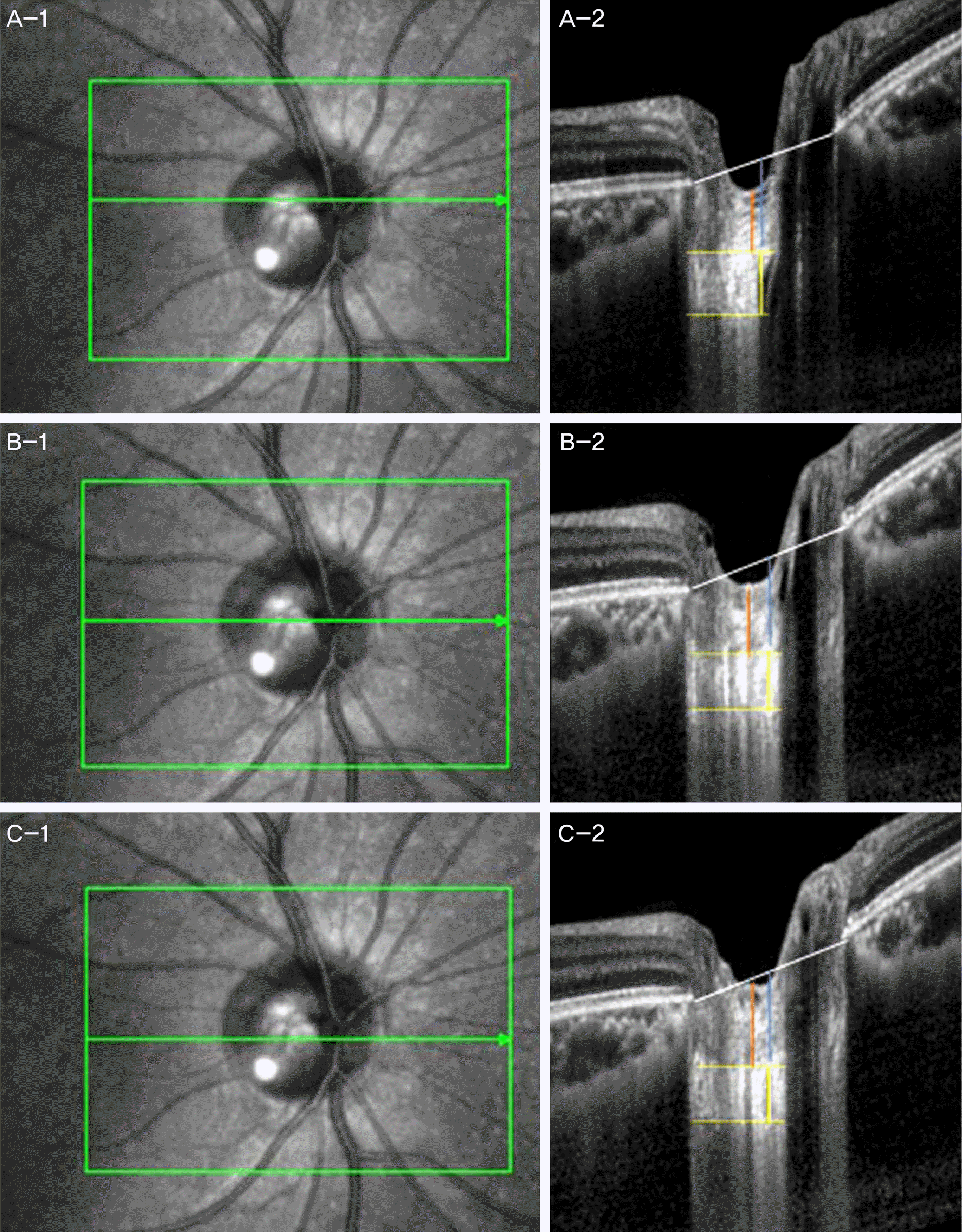 | Figure 1.En face (A-1, B-1, C-1) and B-scan images at superior midperipheral (A-2), middle (B-2), inferior midperipheral (C-2) level of optic nerve head (white line: line connecting Bruch’s membrane opening, yellow dot line: anterior and posterior margin of lamina cribrosa, yellow line: thickness of lamina cribrosa, red line: thickness of prelaminar tissue, blue line: amount of lamina cribrosa displacement). |
 | Figure 2.Relationships of lamina cribrosa thickness (A), prelaminar tissue thickness (B), and lamina cribrosa displacement (C) with axial length depends on locations in optic nerve head (1 = superior midperipheral, 2 = middle, 3 = inferior midperipheral, 4 = mean). p = relationship of axial length and LCT, PT and ALD by linear mixed effect model. β = regression coefficient; SE = standard error; AXL = axial length; sup = superior midperipheral; mid = middle; inf = inferior midperipheral; LCT = lamina cribrosa thickness; ALD = anterior laminar displacement; PT = prelaminar tissue thickness. |
Table 1.
Baseline characteristics of study group
Table 2.
Measurements of the thickness of lamina cribrosa, prelamina tissue, and anterior laminar displacement depends at each locations in optic disc head




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download