초록
Purpose:
To investigate the prevalence of ocular and systemic disease causing amaurosis fugax and to discuss the ocular and systemic manifestation of each disease.
Methods:
Consecutive patients who had amaurosis fugax were retrospectively studied from 2007 to 2013. Carotid evaluation using Doppler was performed in all patients. Ocular and medical histories were taken and bilateral ophthalmic evaluation performed.
Results:
This study included 35 patients. The mean age of patients was 63 years and 27 patients were male; 29 unilateral and 6 bilateral eyes were involved. Associated systemic disease included hypertension (54.3%) and diabetes mellitus (34.2%). The most frequent cause of amaurosis fugax was retinal artery occlusion (28.6%) followed by ocular ischemic syndrome (22.9%), other vascular diseases (11.4%), and retinal vein occlusion (5.7%). The remaining 31.4% patients with amaurosis fugax had no vascular disease. Clinically significant stenosis of the internal carotid artery was observed in 16 patients (45.7%) and 6 of these patients (37.5%) had retinal artery occlusion disease.
Conclusions:
Prevalence and clinical manifestation of amaurosis fugax is very complex. Patients with transient visual disturbance are at risk for retinal artery occlusion, ocular ischemic syndrome and other diseases which cause visual loss. Therefore, careful history taking and urgent systemic and ophthalmic evaluations should be performed.
Go to : 
References
1. Burde RM. Amaurosis fugax. An overview. J Clin Neuroophthalmol. 1989; 9:185–9.
2. The Amaurosis Fugax Study Group. Current management of amaurosis fugax. Stroke. 1990; 21:201–8.
3. Hayreh SS, Zimmerman MB. Amaurosis fugax in ocular vascular occlusive disorders: prevalence and pathogeneses. Retina. 2014; 34:115–22.
4. Bacigalupi M. Amaurosis Fugax–A Clinical Review. The Internet Journal of Allied Health Sciences and Practice. 2006; 4.
5. Kim NR, Chin HS. Progression of impending central retinal vein occlusion to the ischemic variant following intravitreal bevacizumab. Korean J Ophthalmol. 2010; 24:179–81.

6. Lee DH, Lee SJ, Yoon IN. Clinical progress in impending central retinal vein occlusion. Korean J Ophthalmol. 2010; 24:83–8.

7. Miller FW, Santoro TJ. Nifedipine in the treatment of migraine headache and amaurosis fugax in patients with systemic lupus erythematosus. N Engl J Med. 1984; 311:921.

8. Shaw HE Jr, Osher RH, Smith JL. Amaurosis fugax associated with SC hemoglobinopathy and lupus erythematosus. Am J Ophthalmol. 1979; 87:281–5.

9. Heckmann JG, Gaul C, Neundörfer B, et al. Vasospastic amaurosis fugax. J Neurol Neurosurg Psychiatry. 2003; 74:149.

10. Jehn A, Frank Dettwiler B, Fleischhauer J, et al. Exercise-induced vasospastic amaurosis fugax. Arch Ophthalmol. 2002; 120:220–2.
11. Jo YJ, Yun YJ, Kwag JY, Kim JY. Valsalva maneuver-induced amaurosis fugax. J Korean Ophthalmol Soc. 2010; 51:779–83.

12. Gowers WR. On a case of simultaneous embolism of central retinal and middle cerebral arteries. Lancet. 1875; 106:794–6.
13. Fisher M. Transient monocular blindness associated with hemiplegia. AMA Arch Ophthalmol. 1952; 47:167–203.

14. Fisher CM. Observations of the fundus oculi in transient monocular blindness. Neurology. 1959; 9:333–47.

15. Poole CJ, Ross Russell RW, Harrison P, Savidge GF. Amaurosis fugax under the age of 40 years. J Neurol Neurosurg Psychiatry. 1987; 50:81–4.

16. Tippin J, Corbett JJ, Kerber RE, et al. Amaurosis fugax and ocular infarction in adolescents and young adults. Ann Neurol. 1989; 26:69–77.

17. Kathleen BD, James JC. Practical viewing of the optic disc. 1st ed.Burlington: Butterworth-Heinemann;2003. p. 269–344.
18. Ravits J, Seybold ME. Transient monocular visual loss from nar-row-angle glaucoma. Arch Neurol. 1984; 41:991–3.

19. Sørensen PN. Amaurosis fugax. A unselected material. Acta Ophthalmol (Copenh). 1983; 61:583–8.
20. Houwerzijl EJ, van Haelst PL, van Doormaal JJ, Gans RO. Clinical reasoning and decision-making in practice. A 31-year-old woman with transient monocular blindness and polycythaemia. Ned Tijdschr Geneeskd. 2005; 149:125–31.
21. Hayreh SS, Podhajsky PA, Zimmerman MB. Retinal artery occlusion: associated systemic and ophthalmic abnormalities. Ophthalmology. 2009; 116:1928–36.
22. Wijman CA, Gomes JA, Winter MR, et al. Symptomatic and asymptomatic retinal embolism have different mechanisms. Stroke. 2004; 35:e100–2.

23. Hayreh SS, Podhajsky P. Ocular neovascularization with retinal vascular occlusion. II. Occurrence in central and branch retinal artery occlusion. Arch Ophthalmol. 1982; 100:1585–96.
Go to : 
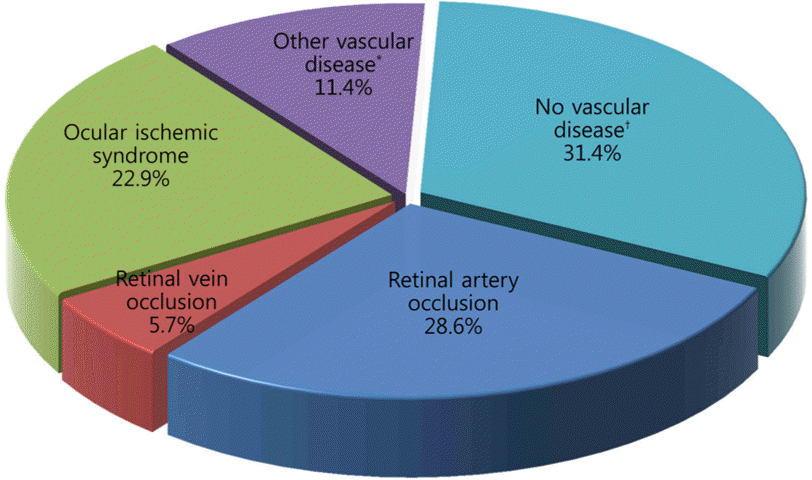 | Figure 1.Disease distribution of new patients with amaurosis fugax. * Diabetic retinopathy, atrial fibrillation; † Transient ischemic attack, migrane, panuveitis. |
Table 1.
Basic characteristics of patients with amaurosis fugax (n = 35)
| Results | |
|---|---|
| Sex (M:F) | 27:8 |
| Age at initial visit (years, range) | 63.77 ± 12.59 (30-83) |
| Eye involved (n, %) | |
| Unilateral | 29 (82.9) |
| Bilateral | 6 (17.1) |
| Underlying disease | |
| Hypertension | 19 (54.3) |
| Diabetes mellitus | 12 (34.3) |
| Cardiovascular disease* | 5 (14.3) |
Table 2.
Carotid doppler findings of the patients with amaurosis fugax
| Retinal artery occlusion (n = 10) | Retinal vein occlusion (n = 2) | Ocular ischemic syndrome (n = 8) | Other vascular disease (n = 4) | No vascular disease (n = 11) | |
|---|---|---|---|---|---|
| Clinically significant stenosis (%)* | 6 (60) | 0 | 6 (75) | 2 (50) | 2 (18.2) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download