초록
Purpose:
To investigate the relationship between changes of corneal epithelium and subbasal nerves in non-Sjögren dry eye using in vivo confocal microscope (IVCM) and self-reported clinical symptoms.
Methods:
The present study included 40 patients with dry eye and 18 healthy control subjects. The dry eye group underwent an evaluation of dry eye symptoms using visual analogue scale (VAS) score and was subdivided into 2 groups; score 0-5 as the low VAS score (LVS) group and score 6 - 10 as the high VAS score (HVS) group. The tear film break-up time, fluorescein staining, Schirmer test and microstructural imaging of epithelium, and subbasal nerve at cornea center with IVCM were performed on both eyes of each patient. Twenty-three normal eyes and 54 eyes of dry eye patients were included in the study. Cell densities and morphological characteristics were analyzed using ImageJ and NeuronJ softwares.
Results:
Both LVS and HVS groups had decreased cell density of superficial, intermediate, and basal epithelium ( p < 0.001). Intermediate epithelial cells were more decreased in the dry eye group with more severe symptoms ( p < 0.0001). Subbasal nerve density ( p < 0.005) was more decreased and nerve beadings, tortuosity, and reflectivity increased in the HVS group than both LVS and control groups ( p < 0.05).
Go to : 
References
1. Uchino M, Schaumberg DA, Dogru M, et al. Prevalence of dry eye disease among Japanese visual display terminal users. Ophthalmology. 2008; 115:1982–8.

2. Guo B, Lu P, Chen X, et al. Prevalence of dry eye disease in Mongolians at high altitude in China: the Henan eye study. Ophthalmic Epidemiol. 2010; 17:234–41.

3. Kim WJ, Kim HS, Kim MS. Current trends in the recognition and treatment of dry eye: a survey of ophthalmologists. J Korean Ophthalmol Soc. 2007; 48:1614–22.

4. Schiffman RM, Walt JG, Jacobsen G, et al. Utility assessment among patients with dry eye disease. Ophthalmology. 2003; 110:1412–9.

5. Buchholz P, Steeds CS, Stern LS, et al. Utility assessment to measure the impact of dry eye disease. Ocul Surf. 2006; 4:155–61.

6. Yamada M, Mizuno Y, Shigeyasu C. Impact of dry eye on work productivity. Clinicoecon Outcomes Res. 2012; 4:307–12.

7. Uchino M, Uchino Y, Dogru M, et al. Dry eye disease and work productivity loss in visual display users: the Osaka study. Am J Ophthalmol. 2014; 157:294–300.

8. Cho BJ, Lee JH, Shim OJ. The relation between clinical manifes-tations of dry eye patients and their BUTs. J Korean Ophthalmol Soc. 1992; 33:297–302.
9. Zhang X, Chen Q, Chen W, et al. Tear dynamics and corneal confocal microscopy of subjects with mild self-reported office dry eye. Ophthalmology. 2011; 118:902–7.

10. Cavanagh HD, Jester JV, Essepian J, et al. Confocal microscopy of the living eye. CLAO J. 1990; 16:65–73.
11. Alhatem A, Cavalcanti B, Hamrah P. In vivo confocal microscopy in dry eye disease and related conditions. Semin Ophthalmol. 2012; 27:138–48.
12. Qazi Y, Aggarwal S, Hamrah P. Image-guided evaluation and monitoring of treatment response in patients with dry eye disease. Graefes Arch Clin Exp Ophthalmol. 2014; 252:857–72.

13. Benítez del Castillo JM, Wasfy MA, Fernandez C, Garcia-Sanchez J. An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Invest Ophthalmol Vis Sci. 2004; 45:3030–5.
14. Tuominen IS, Konttinen YT, Vesaluoma MH, et al. Corneal in-nervation and morphology in primary Sjögren's syndrome. Invest Ophthalmol Vis Sci. 2003; 44:2545–9.
15. Zhang M, Chen J, Luo L, et al. Altered corneal nerves in aqueous tear deficiency viewed by in vivo confocal microscopy. Cornea. 2005; 24:818–24.

16. Villani E, Galimberti D, Viola F, et al. The cornea in Sjogren's syndrome: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2007; 48:2017–22.

17. Labbé A, Alalwani H, Van Went C, et al. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci. 2012; 53:4926–31.

18. Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria pro-posed by the American-European Consensus Group. Ann Rheum Dis. 2002; 61:554–8.
19. Oliveira-Soto L, Efron N. Morphology of corneal nerves using confocal microscopy. Cornea. 2001; 20:374–84.

20. Wang J, Palakuru JR, Aquavella JV. Correlations among upper and lower tear menisci, noninvasive tear break-up time, and the Schirmer test. Am J Ophthalmol. 2008; 145:795–800.

21. Hoşal BM, Ornek N, Zilelioğlu G, Elhan AH. Morphology of corneal nerves and corneal sensation in dry eye: a preliminary study. Eye (Lond). 2005; 19:1276–9.

22. Shim J, Park C, Lee HS, et al. Change in prostaglandin expression levels and synthesizing activities in dry eye disease. Ophthalmology. 2012; 119:2211–9.

23. Parra A, Madrid R, Echevarria D, et al. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat Med. 2010; 16:1396–9.

24. Labbé A, Liang Q, Wang Z, et al. Corneal nerve structure and function in patients with non-sjogren dry eye: clinical correlations. Invest Ophthalmol Vis Sci. 2013; 54:5144–50.
Go to : 
 | Figure 1.
In vivo confocal images of cornea. Superficial epithelium. Compared with (A), (B), and (C) had more decreased cell density. (A) Showing regularly arranged cells with dark nuclei. (B) and (C) showing squamous metaplasia, hyperreflectivity as compared to control. Intermediate epithelium. Compared with (D) and (E), (F) had more decreased cell density. Basal epithelium. Compared with (G), (H) and (I) had more decreased cell density. Bar, 100 μ m. LVS = low visual analogue scale score; HVS = high visual analogue scale score. |
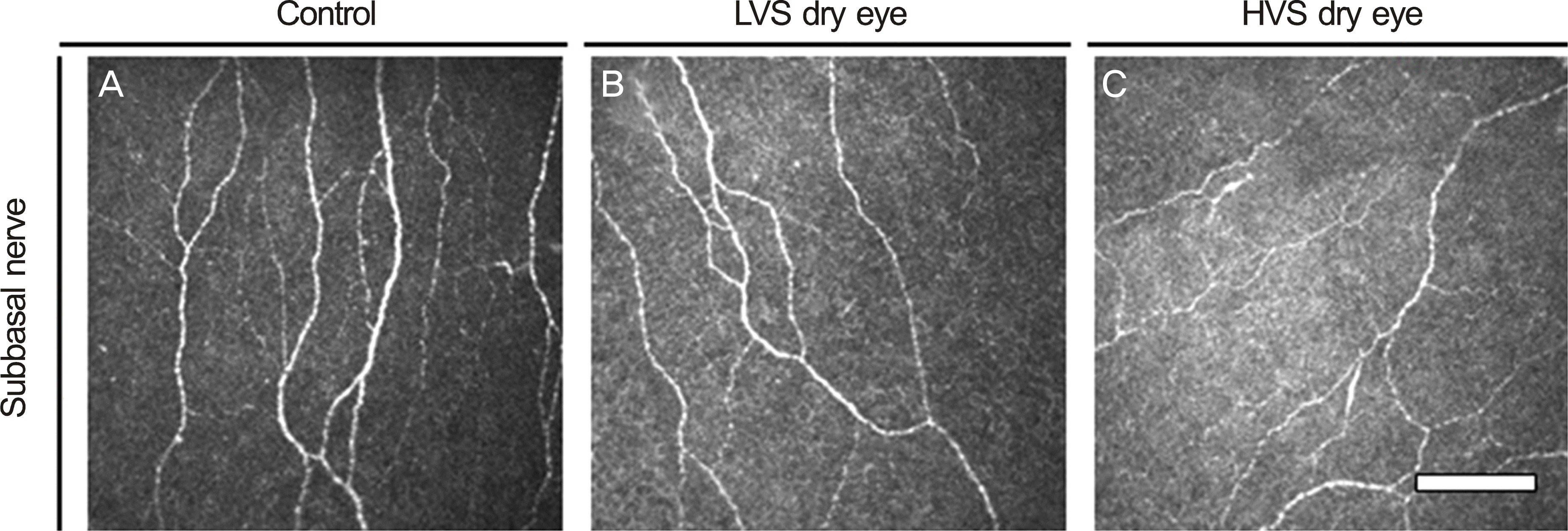 | Figure 2.
In vivo confocal images of cornea. Subbasal nerve. Compared with (A) and (B), (C) had more decreased subbasal nerve density and had more increased subbasal nerve beadings, tortuosity, and reflectivity. Bar, 100 μ m. LVS = low visual analogue scale score; HVS = high visual analogue scale score. |
Table 1.
Demographic data and clinical test results
| Control | LVS | HVS | p-value | |
|---|---|---|---|---|
| Eyes (n) | 23 | 26 | 28 | N/A |
| Age (years) | 47.87 ± 15.93 | 54.39 ± 11.28 | 50.38 ± 15.62 | 0.37 |
| Sex (M/F) | 7/11 | 4/16 | 9/11 | 0.53 |
| BCVA (log MAR) | 0.02 ± 0.07 | 0.03 ± 0.08 | 0.02 ± 0.05 | 0.21 |
| Duration of dry eye (months) | 41.78 ± 52.76 | 27.11 ± 26.44 | 0.32 | |
| Time of treatment (months) | 18.40 ± 23.06 | 15.28 ± 19.37 | 0.67 | |
| Schirmer test (mm/5 min) | 12.8 ± 2.50 | 6.00 ± 1.41 | 5.13 ± 0.89 | <0.001*,†; 0.44‡ |
| FTBUT (s) | 7.5 ± 2.64 | 3.00 ± 0.69 | 1.94 ± 1.00 | <0.001*,†, <0.05‡ |
| Oxford scheme grade | 0.13 ± 0.34 | 1.61 ± 1.24 | 1.94 ± 0.92 | 0.06*, <0.05†, 0.14‡ |
| VAS score | 0.17 ± 0.49 | 2.56 ± 1.42 | 7.25 ± 1.00 | <0.001*,†,‡ |
Values are presented as mean ± SD unless otherwise indicated; Data in parentheses are 95% confidence interval; p-value is by ANOVA.
Table 2.
Cell densities of corneal superficial, intermediate, and basal epithelium by IVCM analysis
|
Epithelium |
|||
|---|---|---|---|
| superficial (cells/mm²) | Intermediate (cells/mm²) | Basal (cells/mm²) | |
| Control | 1,234 ± 149 | 6,931 ± 126 | 11,478 ± 586 |
| LVS | 864 ± 51 | 5,832 ± 232 | 9,023 ± 222 |
| HVS | 789 ± 31 | 5,316 ± 265 | 8,688 ± 209 |
| p-value | <0.001*,†; 0.17‡ | <0.0001*,†,‡ | <0.001*,†; 0.06‡ |
Table 3.
Density and morphological characteristics of corneal subbasal nerve by IVCM analysis
|
Subbasal nerve |
||||
|---|---|---|---|---|
| Density (μ m/mm²) | Beading (No./mm) | Tourtuosity (grade, 0-4) | Reflectivity (grade, 0-4) | |
| Control | 12,030.05 ± 2,203.55 | 178.70 ± 27.39 | 1.6 ± 0.6 | 2.3 ± 0.6 |
| LVS | 10,434.00 ± 2,347.73 | 302.72 ± 18.04 | 3.0 ± 0.6 | 2.8 ± 0.5 |
| HVS | 9,884.56 ± 2,548.56 | 329.38 ± 24.13 | 3.7 ± 0.5 | 3.0 ± 0.4 |
| p-value | <0.005*,†,‡ | <0.001*,†; <0.01‡ | <0.001*,†; <0.05‡ | <0.05*,†; 0.72‡ |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download