초록
Purpose:
The purpose of this study was to investigate the clinical efficacy of Cutanplast® nasal packing after endonasal dacryocystorhinostomy.
Methods:
The present study included a total of 76 adult patients (98 eyes) with primary acquired nasolacrimal duct obstruction who underwent endonasal dacryocystorhinostomy. Fifty-four eyes were packed with Cutanplast® and 44 eyes were packed with Merocel®. Patient discomfort while the packing was in situ, degree of bleeding during the day after operation, functional and anatomical success rate, and postoperative complications such as synechiae, granulation, wound healing (osteal mucosal epithelium epithelization), and revision rate were compared between the packing materials.
Results:
The Cutanplast® was significantly more comfortable and effective at preventing hemorrhage after endonasal dacryocystorhinostomy during the day following the operation. There was no significant difference between the two groups in postoperative anatomical and functional surgical success rate at 1 week, 1 month and 3 months. In comparison with postoperative complications, the Cutanplast® group showed a lower incidence of delayed wound healing (delayed epithelialization of the osteal mucosal epithelium) than the Merocel® group, whereas there was no difference in granulation, synechiae, or revision rate.
Conclusions:
The Cutanplast® nasal pack resulted in significantly less discomfort and less bleeding compared to the Merocel® nasal pack. Moreover, the Cutanplast® showed a lower proportion of delayed wound healing after endonasal dacryocystorhinostomy and had the advantage of low cost compared to other hemostatic nasal packing materials. Therefore, Cutanplast® nasal packing after endonasal dacryocystorhinostomy can be considered a comfortable, cost-effective and clinically-effective method.
Go to : 
References
1. Weber R, Keerl R, Hochapfel F, et al. Packing in endonasal surgery. Am J Otolaryngol. 2001; 22:306–20.

2. Pomerantz J, Dutton JM. Platelet gel for endoscopic sinus surgery. Ann Otol Rhinol Laryngol. 2005; 114:699–704.

3. Shaw CL, Dymock RB, Cowin A, Wormald PJ. Effect of packing on nasal mucosa of sheep. J Laryngol Otol. 2000; 114:506–9.

4. Chandra RK, Kern RC. Advantages and disadvantages of topical packing in endoscopic sinus surgery. Curr Opin Otolaryngol Head Neck Surg. 2004; 12:21–6.

5. Valentine R, Wormald PJ, Sindwani R. Advances in absorbable bi-omaterials and nasal packing. Otolaryngol Clin North Am. 2009; 42:813–28. ix.

6. Cenni E, Ciapetti G, Stea S, et al. Biocompatibility and performance in vitro of a hemostatic gelatin sponge. J Biomater Sci Polym Ed. 2000; 11:685–99.
7. Hajosch R, Suckfuell M, Oesser S, et al. A novel gelatin sponge for accelerated hemostasis. J Biomed Mater Res B Appl Biomater. 2010; 94:372–9.

8. Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health. 1990; 13:227–36.

9. Munk PL, Lin DT, Morris DC. Epiphora: treatment by means of dacryocystoplasty with balloon dilation of the nasolacrimal drainage apparatus. Radiology. 1990; 177:687–90.

10. Konuk O, Kurtulmusoglu M, Knatova Z, Unal M. Unsuccessful lacrimal surgery: causative factors and results of surgical management in a tertiary referral center. Ophthalmologica. 2010; 224:361–6.

11. Mehta U, Huber TC, Sindwani R. Patient expectations and recovery following endoscopic sinus surgery. Otolaryngol Head Neck Surg. 2006; 134:483–7.

12. Weber RK. Nasal packing and stenting. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2009; 8:Doc02.
13. Hesham A, Ghali A. Rapid Rhino versus Merocel nasal packs in septal surgery. J Laryngol Otol. 2011; 125:1244–6.

14. Kim YS, Kim YH, Kim NH, et al. A prospective, randomized, single-blinded controlled trial on biodegradable synthetic polyur-ethane foam as a packing material after septoplasty. Am J Rhinol Allergy. 2011; 25:e77–9.

15. Vaiman M, Eviatar E, Segal S. Effectiveness of second-generation fibrin glue in endonasal operations. Otolaryngol Head Neck Surg. 2002; 126:388–91.

16. Weber R, Hochapfel F, Draf W. Packing and stents in endonasal surgery. Rhinology. 2000; 38:49–62.
17. Shoman N, Gheriani H, Flamer D, Javer A. Prospective, dou-ble-blind, randomized trial evaluating patient satisfaction, bleeding, and wound healing using biodegradable synthetic polyur-ethane foam (NasoPore) as a middle meatal spacer in functional endoscopic sinus surgery. J Otolaryngol Head Neck Surg. 2009; 38:112–8.
18. Field FK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994; 167(1A):2S–6S.

Go to : 
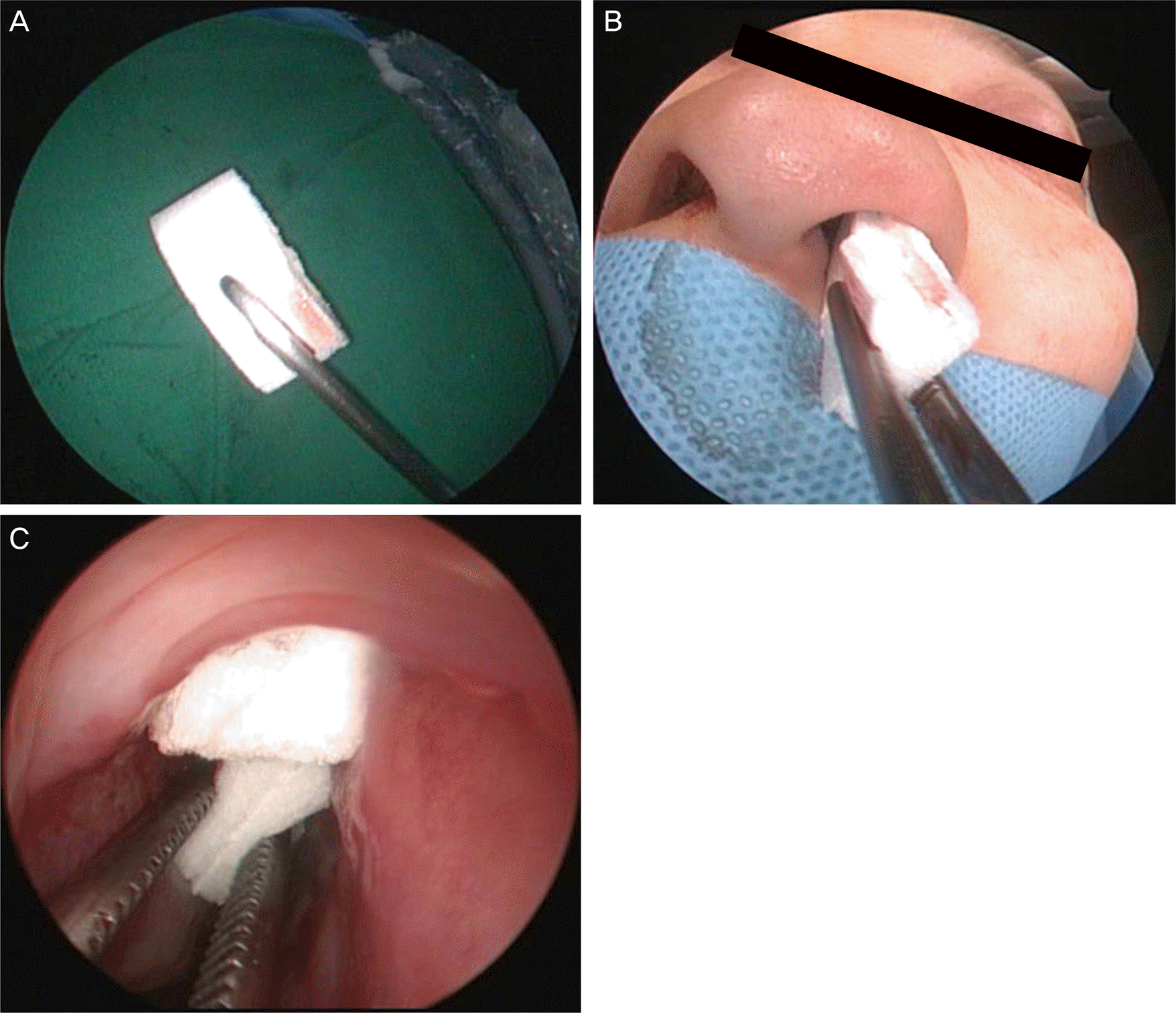 | Figure 1.(A) The Cutanplast® is prepared by dividing it into four pieces. (B) Cutanplast® folded in half is inserted through the nose and (C) packed into the middle meatus. Several Cutanplast® are further inserted. |
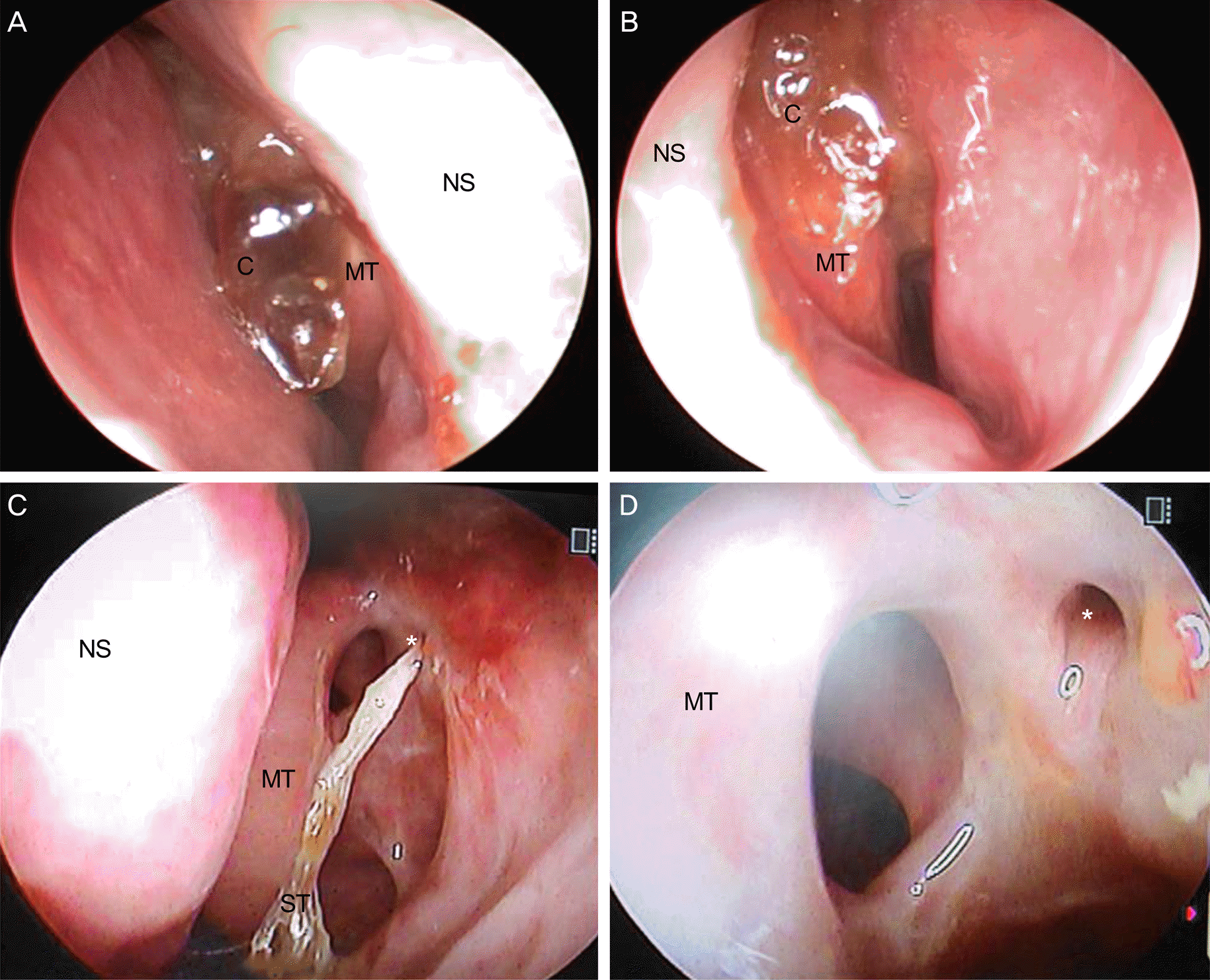 | Figure 2.Endoscopic findings of the middle meatus after endonasal dacryocystorhinostomy with Cutanplast® packing. (A), (B) Liquefied hemostatic gelatin sponge (Cutanplast®) 1 week after application and can be gently removed by a suc-tion device. (C) Normal epithelization of the osteal mucosal epithelium with a silicone tube at 2 months. (D) Normally recovered epithelization of the osteal mucosal epithelium when the silicone tube removed at 3 months. C = liquefied Cutanplast®; NS = nasal septum; MT = middle turbinate; ST = silicone tube. * Osteal opening. |
Table 1.
Postoperative bleeding day after surgery
Table 2.
Munk’s score
Table 3.
Patient demographics and characteristics at baseline
| Merocel® | Cutanplast® | p-value | |
|---|---|---|---|
| Age (years) | 56.4 ± 10.6 | 55.1 ± 8.5 | 0.561* |
| Sex (n, %) | 0.617† | ||
| Male | 5 (14.7) | 8 (19.0) | |
| Female | 29 (85.3) | 34 (81.0) | |
| Side (n, %) | 0.888† | ||
| OD | 23 (52.3) | 29 (53.7) | |
| OS | 21 (47.7) | 25 (46.3) | |
| Previous dacryocystitis history (n, %) | 1 (2.27) | 1 (1.9) | 0.883† |
| Mean time to tube removal (weeks) | 11.8 ± 1.1 | 11.7 ± 1.2 | 0.629* |
Table 4.
Comparision of postoperative discomfort, bleeding, Munk’s score
| Type |
VAS/Grade |
Mean ± SD | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||||
| Discomfort during insertion | Merocel® | 0 | 3 | 21 | 9 | 1 | 2.05 ± 0.86 | 0.00* |
| Cutanplast® | 14 | 20 | 16 | 4 | 0 | 1.13 ± 0.62 | ||
| Postoperative bleeding day after surgery | Merocel® | 0 | 5 | 20 | 14 | 5 | 1.98 ± 0.70 | 0.00* |
| Cutanplast® | 1 | 22 | 25 | 4 | 1 | 0.65 ± 0.59 | ||
| Type |
Munk’s score |
Mean ± SD | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||||
| Postoperative 1 week | Merocel® | 16 | 21 | 4 | 3 | 0 | 0 | 0.86 ± 0.85 | 0.423* |
| Cutanplast® | 25 | 23 | 3 | 2 | 1 | 0 | 0.72 ± 0.88 | ||
| Postoperative 1 month | Merocel® | 15 | 20 | 4 | 2 | 3 | 0 | 1.05 ± 1.1 | 0.278* |
| Cutanplast® | 25 | 19 | 6 | 3 | 1 | 0 | 0.81 ± 0.97 | ||
| Postoperative 3 months | Merocel® | 16 | 16 | 6 | 4 | 2 | 0 | 1.09 ± 1.1 | 0.401* |
| Cutanplast® | 23 | 19 | 7 | 4 | 1 | 0 | 0.91 ± 1.0 | ||
Table 5.
Comparision of functional success rate and anatomical success rate
|
Functional success rate |
Anatomical success rate |
|||||
|---|---|---|---|---|---|---|
| Merocel® | Cutanplast® | p-value | Merocel® | Cutanplast® | p-value | |
| Postoperative 1 week (%) | 41/44 (93.18) | 51/54 (94.44) | 0.795* | 42/44 (95.45) | 51/54 (94.44) | 0.821* |
| Postoperative 1 month (%) | 39/44 (88.64) | 50/54 (92.59) | 0.500* | 41/44 (93.18) | 51/54 (94.44) | 0.795* |
| Postoperative 3 months (%) | 38/44 (86.36) | 49/54 (90.74) | 0.495* | 41/44 (93.18) | 50/54 (92.60) | 0.910* |
Table 6.
Comparison of postoperative general complication
| Merocel® | Cutanplast® | p-value | |
|---|---|---|---|
| Granuloma formation (n, %) | 12 (27.3) | 13 (24.7) | 0.718* |
| Synechiae (n, %) | 2 (4.5) | 1 (1.9) | 0.441* |
| Delayed wound healing (delayed epithelialization) (n, %) | 5 (11.36) | 1 (1.82) | 0.051* |
| Revision (n, %) | 3 (6.82) | 1 (1.82) | 0.217* |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download