Abstract
Purpose
To evaluate the efficacy and safety of primary intravitreal bevacizumab injection in stage 3 retinopathy of prematurity with plus signs.
Methods
We reviewed retrospectively the medical records of 30 eyes of 16 patients diagnosed with stage 3 retinopathy of pre-maturity with plus signs treated with primary intravitreal bevacizumab injection between March 1, 2011 and February 28, 2013 and followed up for at least 9 months.
Results
Mean gestational age was 26 + 4 weeks ± 11 days and mean birth weight was 822 ± 251.4 g. The locations of disease were zone II in 24 eyes and zone III in 6 eyes. Intravitreal bevacizumab injection was performed after the mean 1.3 ± 1 day after plus signs were detected. Mean postconceptional age at treatment was 38 + 2 weeks ± 16 days. Mean follow-up period was 16.6 ± 6.9 months. Plus signs started to regress after the mean 4.6 ± 2.3 days after injection and completely regressed after the mean 24.3 ± 12.4 days. Cataract extraction was performed in 1 eye due to a cataract that appeared not associated with the injection procedure, but was regarded as a treatment failure. There were no local or systemic complications.
Go to : 
References
1. Terry TL. Fibroblastic Overgrowth of Persistent Tunica Vasculosa Lentis in Infants Born Prematurely: II. Report of Cases-Clinical Aspects. Trans Am Ophthalmol Soc. 1942; 40:262–84.
2. Darlow BA, Hutchinson JL, Henderson-Smart DJ. . Prenatal risk factors for severe retinopathy of prematurity among very preterm infants of the Australian and New Zealand Neonatal Network. Pediatrics. 2005; 115:990–6.

3. Anderson CG, Benitz WE, Madan A. Retinopathy of prematurity and pulse oximetry: a national survey of recent practices. J Perinatol. 2004; 24:164–8.

4. SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Carlo WA, Finer NN. . Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010; 362:1959–69.

5. Tompkins C. A sudden rise in the prevalence of retinopathy of prematurity blindness? Pediatrics. 2001; 108:526.

6. Gibson DL, Sheps SB, Schechter MT. . Retinopathy of pre-maturity: a new epidemic? Pediatrics. 1989; 83:486–92.

7. Smith LE. Through the eyes of a child: understanding retinopathy through ROP the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2008; 49:5177–82.

8. Das A, McGuire PG. Retinal and choroidal angiogenesis: patho-physiology and strategies for inhibition. Prog Retin Eye Res. 2003; 22:721–48.

9. Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of pre-maturity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003; 121:1684–94.
10. Good WV. Early Treatment for Retinopathy of Prematurity Cooperative Group. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 2004; 102:233–48. discussion 248-50.
11. Hurwitz H, Fehrenbacher L, Novotny W. . Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004; 350:2335–42.

12. CATT Research Group, Martin DF, Maguire MG. . Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011; 364:1897–908.

13. Tonello M, Costa RA, Almeida FP. . Panretinal photo-coagulation versus PRP plus intravitreal bevacizumab for high-risk proliferative diabetic retinopathy (IBeHi study). Acta Ophthalmol. 2008; 86:385–9.

14. Michaelides M, Kaines A, Hamilton RD. . A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010; 117:1078–86.e2.
15. Epstein DL, Algvere PV, von Wendt G. . Bevacizumab for macular edema in central retinal vein occlusion: a prospective, randomized, double-masked clinical study. Ophthalmology. 2012; 119:1184–9.

16. Higashiyama T, Sawada O, Kakinoki M. . Prospective comparisons of intravitreal injections of triamcinolone acetonide and bevacizumab for macular oedema due to branch retinal vein occlusion. Acta Ophthalmol. 2013; 91:318–24.

17. International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005; 123:991–9.
18. Multicenter trial of cryotherapy for retinopathy of prematurity. One-year outcome--structure and function. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol. 1990; 108:1408–16.
19. Sato T, Kusaka S, Shimojo H, Fujikado T. Vitreous levels of erythropoietin and vascular endothelial growth factor in eyes with retinopathy of prematurity. Ophthalmology. 2009; 116:1599–603.

20. Lee JY, Chae JB, Yang SJ. . Effects of intravitreal bevacizumab and laser in retinopathy of prematurity therapy on the development of peripheral retinal vessels. Graefes Arch Clin Exp Ophthalmol. 2010; 248:1257–62.

21. Choi W, Heo H. Effect of laser photocoagulation and intravitreal bevacizumab injection on zone I retinopathy of prematurity. J Korean Ophthalmol Soc. 2012; 53:120–6.

22. Mintz-Hittner HA, Kennedy KA, Chuang AZ; BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011; 364:603–15.

23. Jager RD, Aiello LP, Patel SC, Cunningham ET Jr. Risks of intra-vitreous injection: a comprehensive review. Retina. 2004; 24:676–98.

24. Kodjikian L, Souied EH, Mimoun G. . Ranibizumab versus Bevacizumab for Neovascular Age-related Macular Degeneration: Results from the GEFAL Noninferiority Randomized Trial. Ophthalmology. 2013; 120:2300–9.

25. Wu WC, Yeh PT, Chen SN. . Effects and complications of bevacizumab use in patients with retinopathy of prematurity: a multicenter study in taiwan. Ophthalmology. 2011; 118:176–83.

26. Mintz-Hittner HA, Kuffel RR Jr. Intravitreal injection of bev-acizumab (avastin) for treatment of stage 3 retinopathy of prematurity in zone I or posterior zone II. Retina. 2008; 28:831–8.

27. Chung EJ, Kim JH, Ahn HS, Koh HJ. Combination of laser photo-coagulation and intravitreal bevacizumab (Avastin) for aggressive zone I retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2007; 245:1727–30.

28. Sato T, Wada K, Arahori H. . Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol. 2012; 153:327–33.e1.

29. Karaca C, Oner AO, Mirza E. . Bilateral effect of unilateral bevacizumab injection in retinopathy of prematurity. JAMA Ophthalmol. 2013; 131:1099–101.

30. Larsen JS. The sagittal growth of the eye. 3. Ultrasonic measurement of the posterior segment (axial length of the vitreous) from birth to puberty. Acta Ophthalmol (Copenh). 1971; 49:441–53.
Go to : 
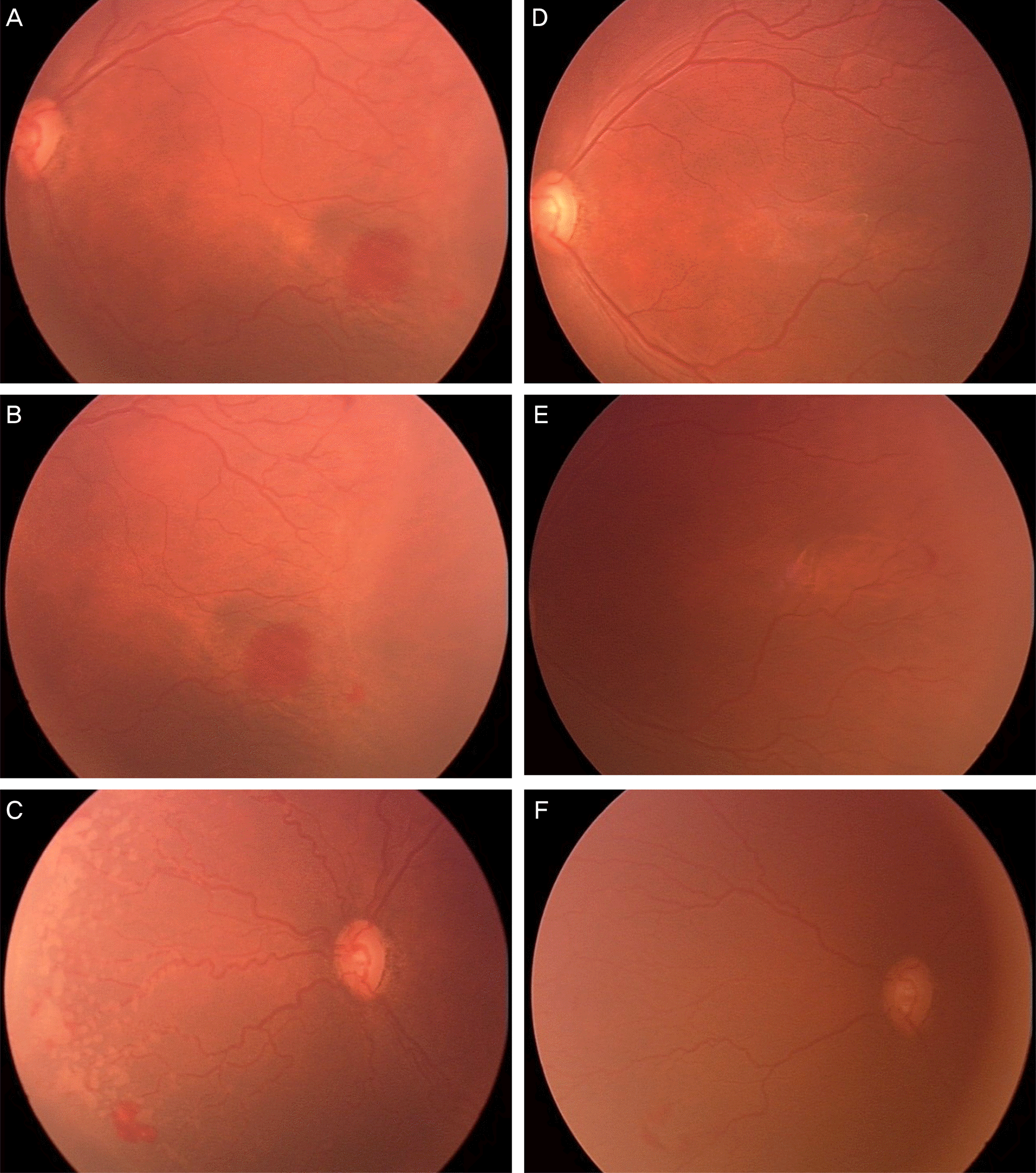 | Figure 1.Case 15. Fundus photographs taken just before operation (A, B, C) and one month after intravitreal bevacizumab injection (D, E, F). (A, B, C) Zone II, stage III retinopathy of prematurity with plus signs. (D, E, F) Regressed plus signs with decreased vascular tortuousness and disappeared retinal neovascularization and hemorrhage are noted. Further vascularization in zone II is also noted. |
Table 1.
Characteristics of study population (30 eyes of 16 patients)
Table 2.
Characteristics of individual eye treated with primary intravitreal bevacizumab injection for stage 3 + retinopathy of pre-maturity




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download