초록
Purpose:
To evaluate whether intraocular pressure reduction by intravenous injection of mannitol before phacoemulsification‐ cataract surgery can have a protective effect on corneal endothelium.
Methods:
Patients undergoing sequential bilateral cataract surgery were divided into 2 groups, 36 eyes with anterior chamber depth (ACD) ˂ 2.50 mm (group A) and 44 eyes with ACD ≥ 2.50 mm (group B). In each group, preoperative intravenous injection of mannitol was performed in 1 randomly selected eye of the patient. The specular microscopic examination including cell density (ECD), coefficient of variation (CV), hexagonality (HA) of corneal endothelium, and corneal thickness was performed on postoperative 1 day, 2 weeks, and 5 weeks. In each group, the parameters were compared between the eyes with mannitolization and the contralateral eyes without mannitolization.
Results:
In group A, eyes with preoperative mannitolization showed significantly higher ECD at postoperative 1 day and 5 weeks and showed a significantly thinner cornea at postoperative 1 day than those without mannitolization (all p < 0.05). However, in group B, there was no significant difference of ECD, CV, HA, and corneal thickness between the eyes with and without mannitolization.
Go to : 
References
1. Jung KI, Yang JW, Lee YC, Kim SY. Cataract surgery in eyes with nanophthalmos and relative anterior microphthalmos. Am J Ophthalmol. 2012; 153:1161–8.e1.

2. Nihalani BR, Jani UD, Vasavada AR, Auffarth GU. Cataract surgery in relative anterior microphthalmos. Ophthalmology. 2005; 112:1360–7.

3. Auffarth GU, Blum M, Faller U, et al. Relative anterior microphthalmos: morphometric analysis and its implications for cataract surgery. Ophthalmology. 2000; 107:1555–60.

4. Lee KM, Lee HS, Kim MS. Clinical results of phacoemulsification in eyes with acute angle-closure glaucoma in the aspect of complications. J Korean Ophthalmol Soc. 2009; 50:44–50.

5. Kirsch RE, Steinman W. Digital pressure, an important safeguard in cataract surgery. AMA Arch Ophthalmol. 1955; 54:697–703.

6. Davidson B, Kratz RP, Mazzocco TR, Maloney WF. An evaluation of the Honan intraocular pressure reducer. J Am Intraocul Implant Soc. 1979; 5:237.

7. Miettinen R, Airaksinen PJ, Pihlajaniemi R, Puhakka K. Preoperative timolol and ocular compression in cataract surgery. Acta Ophthalmol (Copenh). 1982; 60:622–7.

8. Robbins R, Blumenthal M, Galin MA. Reduction of vitreous weight by ocular massage. Am J Ophthalmol. 1970; 69:603–7.

9. Quist LH, Stapleton SS, McPherson SD Jr. Preoperative use of the Honan intraocular pressure reducer. Am J Ophthalmol. 1983; 95:536–8.

10. Chan FM, Lee L. Nanophthalmic cataract extraction. Clin Experiment Ophthalmol. 2004; 32:535–8.
11. Jabs DA, Nussenblatt RB, Rosenbaum JT; Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005; 140:509–16.
12. Naumann GOH, Apple DJ. Pathologie des Auges. Berlin, Heidelberg: Springer-Verlag;1980.
13. Weiss AH, Kousseff BG, Ross EA, Longbottom J. Simple microphthalmos. Arch Ophthalmol. 1989; 107:1625–30.

14. Weiss AH, Kousseff BG, Ross EA, Longbottom J. Complex microphthalmos. Arch Ophthalmol. 1989; 107:1619–24.

15. Parrishll RK, Donaldson K, Kairala MBM, Simmons RJ. Nanophthalmos, Relative Anterior Microphthalmos, and Axial Hyperopia. Steinert RF, editor. Cataract Surgery. 3rd ed.Philadelphia: Saunders;2010. chap. 33.
16. Steijns D, Bijlsma WR, Van der Lelij A. Cataract surgery in patients with nanophthalmos. Ophthalmology. 2013; 120:266–70.

17. Wladis EJ, Gewirtz MB, Guo S. Cataract surgery in the small adult eye. Surv Ophthalmol. 2006; 51:153–61.

18. Faucher A, Hasanee K, Rootman DS. Phacoemulsification and intraocular lens implantation in nanophthalmic eyes: report of a me-dium-size series. J Cataract Refract Surg. 2002; 28:837–42.
19. Hwang JH, Yeom DJ, Kim JS, Lee JH. A case of acute angle-clo-sure glaucoma in a nanophthalmos patient. J Korean Ophthalmol Soc. 2010; 51:303–6.

21. Mandal AK. Cataract surgery with primary posterior chamber intraocular lens implantation in nanophthalmos. Ophthalmic Surg Lasers. 2001; 32:333–5.

22. Kong M, Kim JH, Kim SJ, Kang SW. Full-thickness sclerotomy for uveal effusion syndrome. Korean J Ophthalmol. 2013; 27:294–8.

23. Lee JH, Choi JY, Kim SS. Two cases of uveal effusion syndrome. Korean J Ophthalmol. 2006; 20:124–7.

24. Allingham RR, Damji KF, Freedman SF, et al. Cholinergic Stimulators and Hyperosmotic Agents. Allingham RR, Damji KF, Freedman SF, editors. Shields Textbook of Glaucoma. 6th ed.Philadelphia: Lippincott Williams & Wilkins;2011. chap. 32.
Go to : 
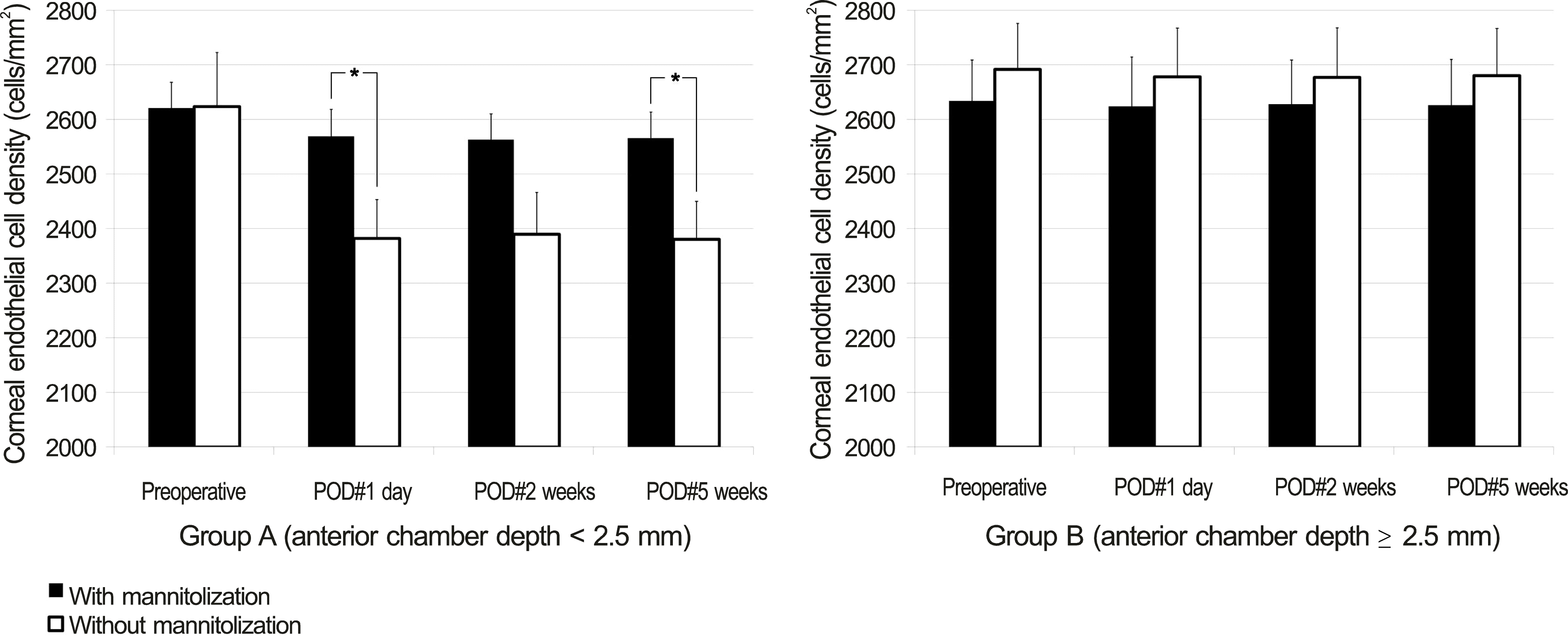 | Figure 1.Comparison of mannitolization on postoperative endothelial cell density (mean ± S.E.M, cells/mm2) between group A (ACD < 2.5 mm) and group B (ACD ≥ 2.5 mm). Student’s t-test was used. * Statistically significant differences among groups ( p < 0.05). SEM = standard error of mean; ACD = anterior chamber depth; POD = postoperative day. |
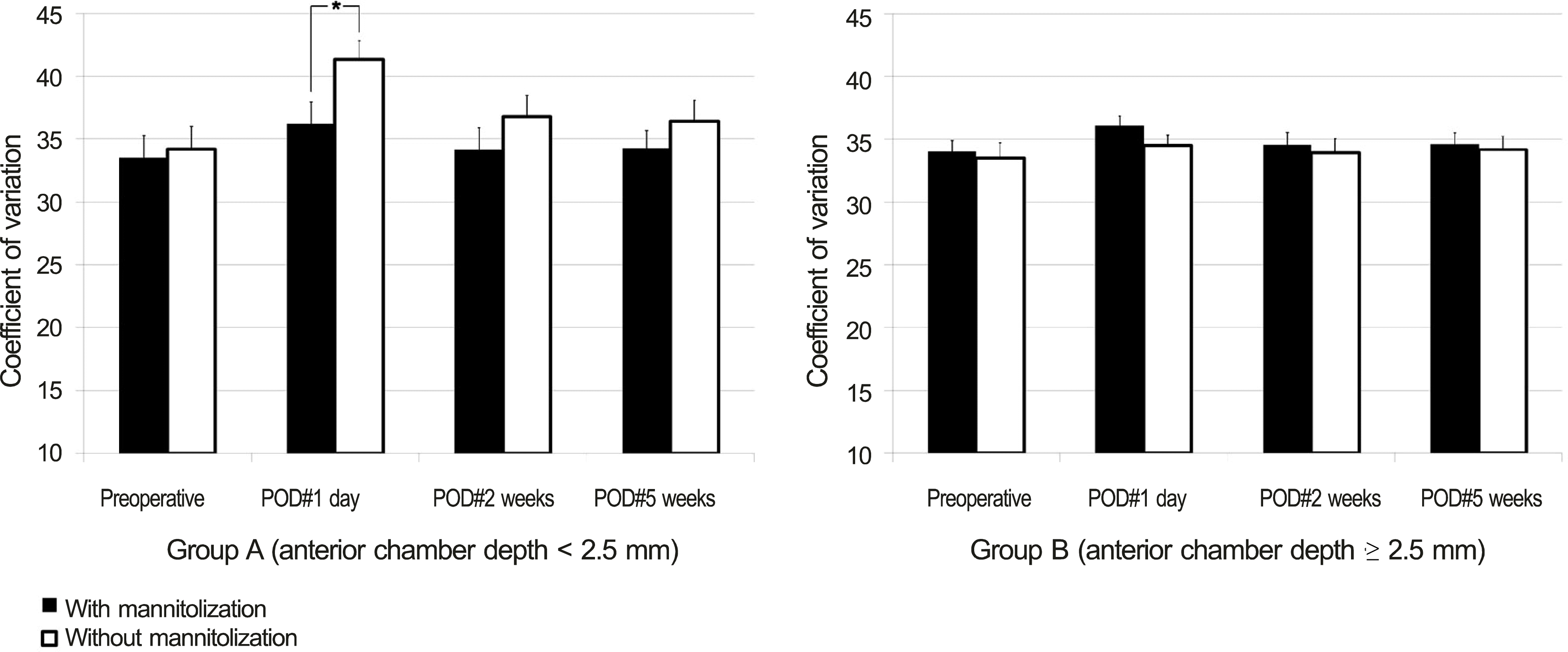 | Figure 2.Comparison of mannitolization on postoperative endothelial cell polymegathism (coefficient of variation, mean ± S.E.M) between group A (ACD < 2.5 mm) and group B (ACD ≥ 2.5 mm). Student’s t-test was used. * Statistically significant differences among groups ( p < 0.05). SEM = standard error of mean; ACD = anterior chamber depth; POD = postoperative day. |
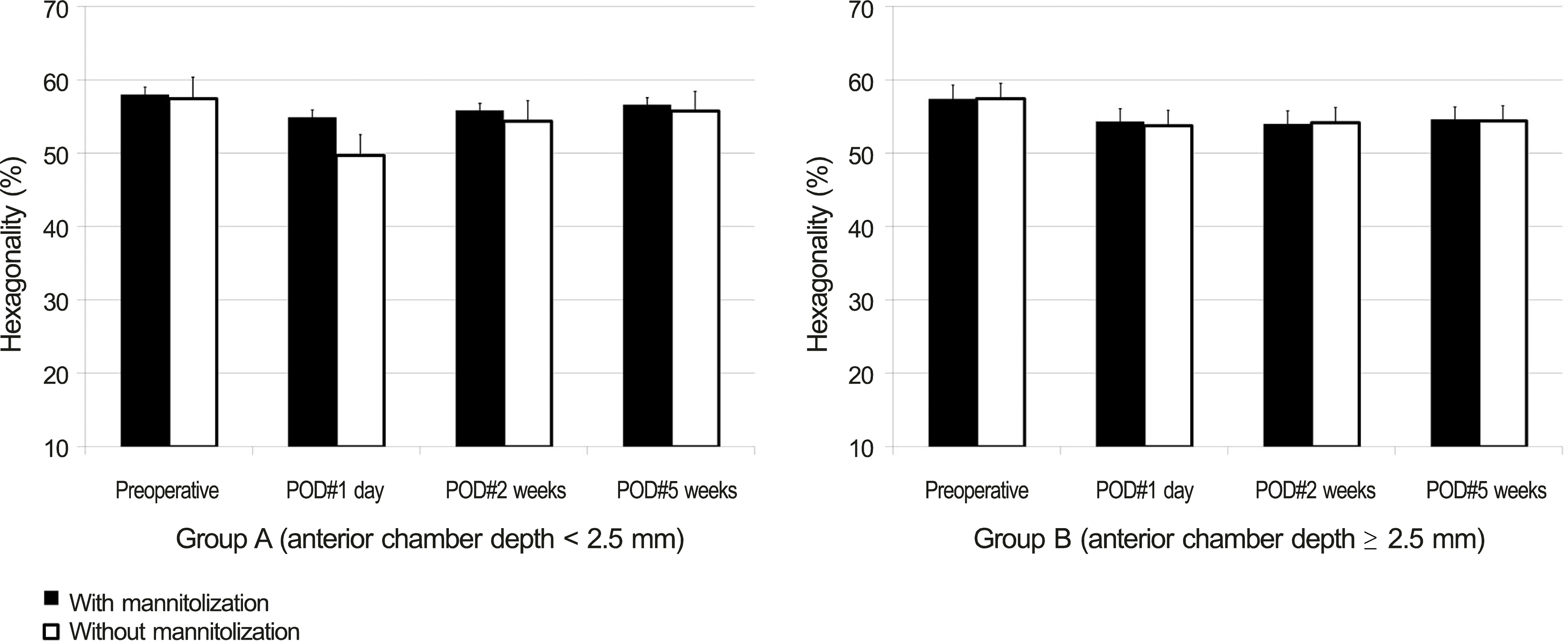 | Figure 3.Comparison of mannitolization on postoperative endothelial cell hexagonality (mean ± S.E.M, %) between group A (ACD < 2.5 mm) and group B (ACD ≥ 2.5 mm). SEM = standard error of mean; ACD = anterior chamber depth; POD = postoperative day. Student’s t-test was used. |
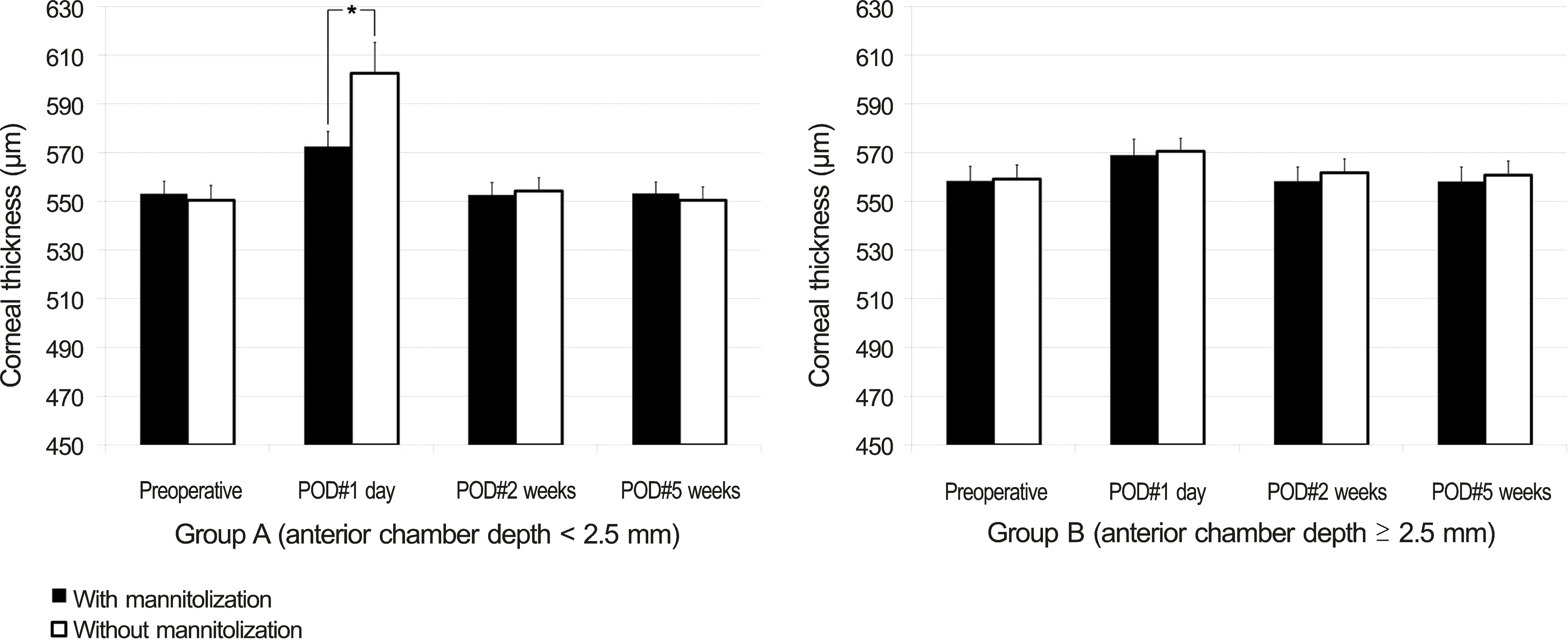 | Figure 4.Comparison of mannitolization on postoperative corneal thickness (mean ± S.E.M, μ m) between group A (ACD < 2.5 mm) and group B (ACD ≥ 2.5 mm). SEM = standard error of mean; ACD = anterior chamber depth; POD = postoperative day. Student’s t-test was used. * Statistically significant differences among groups ( p < 0.05). |
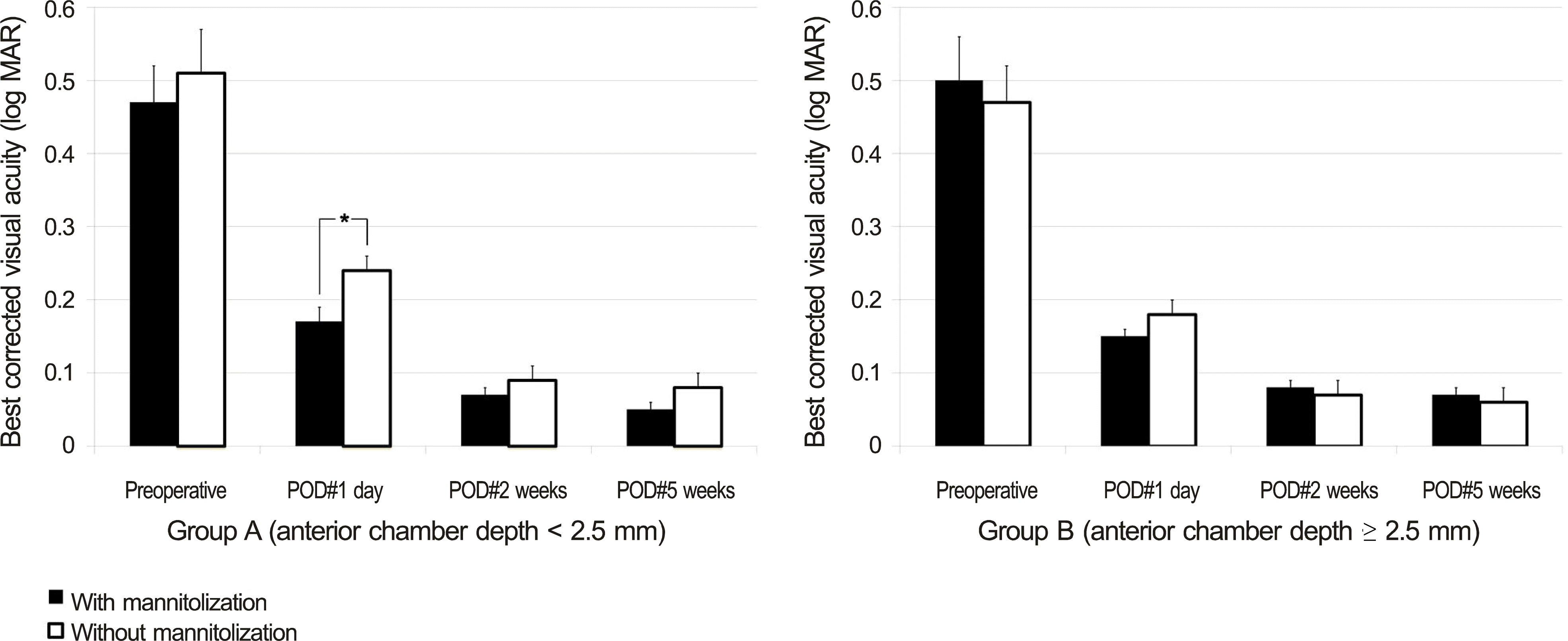 | Figure 5.Comparison of mannitolization on best corrected visual acuity (log MAR, mean ± S.E.M) between group A (ACD < 2.5 mm) and group B (ACD ≥ 2.5 mm). SEM = standard error of mean; ACD = anterior chamber depth; POD = postoperative day. Student’s t-test was used. * Statistically significant differences among groups ( p < 0.05). |
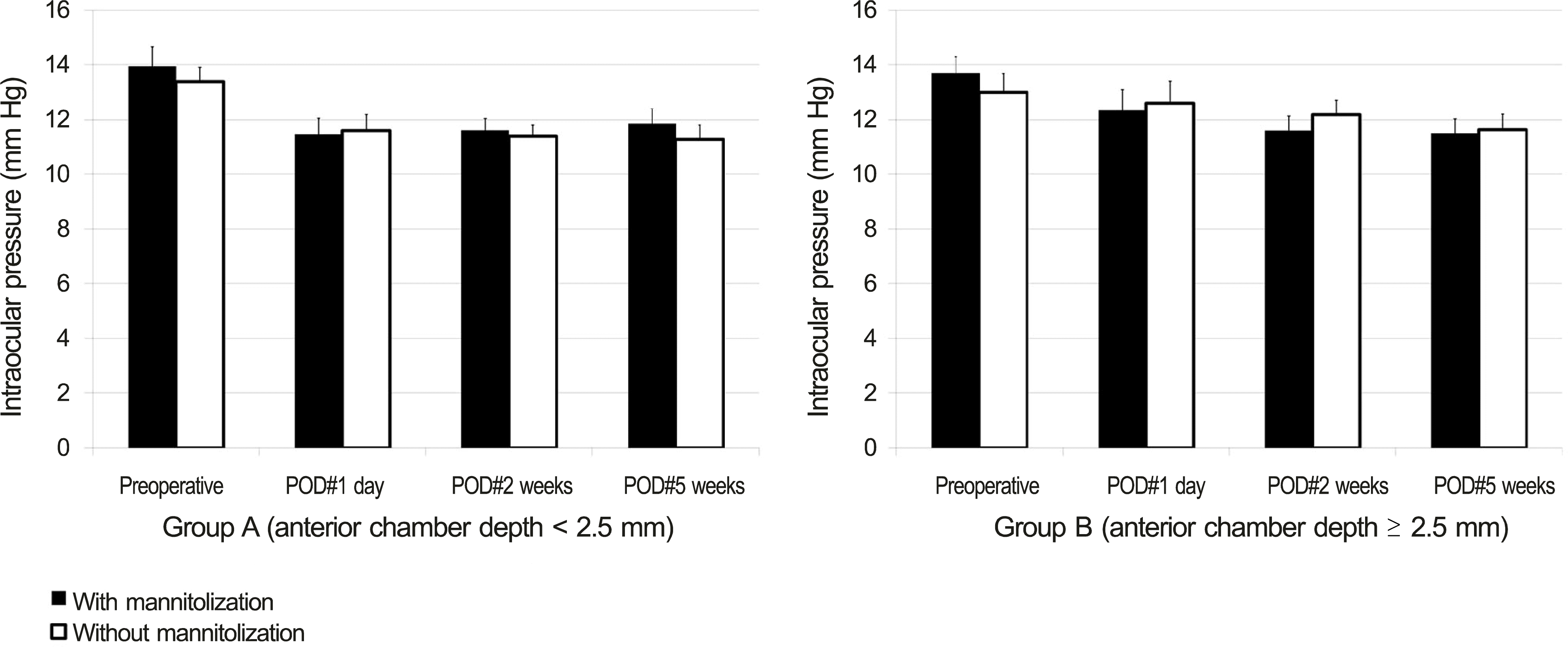 | Figure 6.Comparison of mannitolization on intraocular pressure (mean ± S.E.M, mm Hg) between group A (ACD < 2.5 mm) and group B (ACD ≥ 2.5 mm). SEM = standard error of mean; ACD = anterior chamber depth; POD = postoperative day. Student’s t-test was used. |
Table 1.
Preoperative clinical characteristics of each group according to the ACD
| Characteristic | Group A (ACD < 2.5 mm)(n = 36) | Group B (ACD ≥ 2.5 mm)(n = 44) | p-value | |
|---|---|---|---|---|
| Sex* (number of male:female) | 18:18 | 16:28 | 0.220 | |
| Laterality* (number of right eye:left eye) | 18:18 | 22:22 | 1.000 | |
| Age† (years) | 68.94 ± 0.83 | 70.00 ± 0.80 | 0.364 | |
| BCVA (log MAR)† | 0.49 ± 0.04 | 0.48 ± 0.04 | 0.886 | |
| IOP† (mm Hg) | 13.67 ± 0.45 | 13.34 ± 0.46 | 0.619 | |
| Axial length† (mm) | 22.86 ± 0.09 | 24.07 ± 0.11 | <0.001‡ | |
| ACD† (mm) | 2.16 ± 0.04 | 3.11 ± 0.03 | <0.001‡ | |
| Corneal endothelium | Cell density† (cells/mm2) | 2622.19 ± 54.33 | 2663.16 ± 55.99 | 0.606 |
| Coefficient of variation† | 33.86 ± 1.25 | 33.75 ± 0.74 | 0.939 | |
| Hexagonality† (%) | 57.72 ± 1.54 | 57.41 ± 1.43 | 0.882 | |
| Corneal thickness† (μ m) | 551.81 ± 3.99 | 558.80 ± 4.18 | 0.237 | |
| Nuclear opacity (LOCS III)* | 3.83 ± 0.81 | 3.84 ± 0.81 | 0.997 | |
Table 2.
Comparison of postoperative outcome of each group according to ACD over time
| Group A (ACD < 2.5 mm)(n = 36) | Group B (ACD ≥ 2.5 mm)(n = 44) | p-value | |||
|---|---|---|---|---|---|
| Total operation time* (min) | 11.42 ± 0.67 | 10.50 ± 0.40 | 0.246 | ||
| Phaco energy* | 407.38 ± 29.89 | 412.84 ± 22.29 | 0.884 | ||
| BCVA (log MAR)* | POD #1 day | 0.21 ± 0.02 | 0.16 ± 0.01 | 0.026‡ | |
| POD #2 weeks | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.758 | ||
| POD #5 weeks | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.967 | ||
| IOP* (mm Hg) | POD #1 day | 11.53 ± 0.42 | 12.45 ± 0.56 | 0.187 | |
| POD #2 weeks | 11.50 ± 0.29 | 11.89 ± 0.38 | 0.438 | ||
| POD #5 weeks | 11.56 ± 0.38 | 11.57 ± 0.39 | 0.982 | ||
| Corneal endothelium | Cell density* (cells/mm2) | POD #1 day | 2475.56 ± 45.71 | 2651.41 ± 62.83 | 0.027‡ |
| POD #2 weeks | 2476.44 ± 46.98 | 2652.66 ± 60.44 | 0.024‡ | ||
| POD #5 weeks | 2473.03 ± 44.70 | 2653.41 ± 59.95 | 0.018‡ | ||
| Coefficient of variation* | POD #1 day | 38.75 ± 1.23 | 35.27 ± 0.59 | 0.014‡ | |
| POD #2 weeks | 35.47 ± 1.23 | 34.23 ± 0.75 | 0.390 | ||
| POD #5 weeks | 35.36 ± 1.08 | 34.39 ± 0.70 | 0.437 | ||
| Hexagonality* (%) | POD #1 day | 52.31 ± 1.55 | 54.05 ± 1.35 | 0.398 | |
| POD #2 weeks | 55.11 ± 1.48 | 54.09 ± 1.36 | 0.613 | ||
| POD #5 weeks | 56.19 ± 1.42 | 54.50 ± 1.35 | 0.392 | ||
| Corneal thickness* (μ m) | POD #1 day | 587.56 ± 7.49 | 569.75 ± 4.27 | 0.043‡ | |
| POD #2 weeks | 553.44 ± 3.78 | 560.02 ± 4.09 | 0.250 | ||
| POD #5 weeks | 551.89 ± 3.57 | 559.45 ± 4.17 | 0.183 | ||
| Descemet’s membrane folding† (number of eyes) | POD #1 day | 8 | 7 | 0.472 | |
| Anterior chamber inflammation* | POD #1 day | 1.39 ± 0.10 | 1.28 ± 0.09 | 0.420 | |
Table 3.
Preoperative clinical characteristics between patients with preoperative mannitolization and without preoperative mannitolization in group A (ACD < 2.5 mm) & in group B (ACD ≥ 2.5 mm)
|
Group A (ACD < 2.5 mm) (n = 36) |
Group B (ACD ≥ 2.5 mm) (n = 44) |
||||||
|---|---|---|---|---|---|---|---|
| Mannitol (+)(n = 18) | Mannitol (-)(n = 18) | p-value | Mannitol (+)(n = 18) | Mannitol (-)(n = 18) | p-value | ||
| BCVA (log MAR)* | 0.47 ± 0.05 | 0.51 ± 0.06 | 0.611 | 0.50 ± 0.06 | 0.47 ± 0.05 | 0.759 | |
| IOP* (mm Hg) | 13.94 ± 0.74 | 13.39 ± 0.53 | 0.545 | 13.68 ± 0.63 | 13.00 ± 0.68 | 0.466 | |
| Axial length* (mm) | 22.87 ± 0.14 | 22.84 ± 0.13 | 0.876 | 24.06 ± 0.15 | 24.09 ± 0.16 | 0.887 | |
| ACD* (mm) | 2.14 ± 0.06 | 2.17 ± 0.05 | 0.711 | 3.11 ± 0.05 | 3.11 ± 0.04 | 0.966 | |
| Corneal endothelium | Cell density*(cells/mm2) | 2,620.61 ± 48.06 | 2,623.78 ± 99.22 | 0.977 | 2,634.09 ± 75.17 | 2,692.23 ± 84.30 | 0.609 |
| Coefficient of variation* | 33.50 ± 1.80 | 34.22 ± 1.79 | 0.777 | 34.00 ± 0.88 | 33.50 ± 1.21 | 0.740 | |
| Hexagonality* (%) | 58.00 ± 1.03 | 57.44 ± 2.95 | 0.860 | 57.36 ± 1.95 | 57.45 ± 2.13 | 0.975 | |
| Corneal thickness* (μ m) | 553.11 ± 5.24 | 550.50 ± 6.14 | 0.748 | 558.36 ± 6.08 | 559.23 ± 5.89 | 0.919 | |
| Nuclear opacity (LOCS III)† | 3.83 ± 0.79 | 3.83 ± 0.86 | 0.774 | 3.82 ± 0.80 | 3.86 ± 0.83 | 0.924 | |
| Total operation time* (min) | 10.71 ± 0.76 | 12.12 ± 1.10 | 0.301 | 10.42 ± 0.56 | 10.58 ± 0.60 | 0.843 | |
| Phaco energy* | 406.27 ± 40.75 | 408.49 ± 44.92 | 0.971 | 412.43 ± 25.45 | 413.25 ± 37.24 | 0.986 | |
Table 4.
Comparison of postoperative descemet’s membrane and anterior chamber inflammation between patients with preoperative mannitolization and without preoperative mannitolization in group A (ACD < 2.5 mm) & in group B (ACD ≥ 2.5 mm) at postoperative day 1
|
Group A (ACD < 2.5 mm) (n = 36) |
Group B (ACD ≥ 2.5 mm) (n = 44) |
|||||
|---|---|---|---|---|---|---|
| Mannitol (+)(n = 18) | Mannitol (-)(n = 18) | p-value | Mannitol (+)(n = 22) | Mannitol (-)(n = 22) | p-value | |
| Descemet’s membrane folding*(number of eyes) | 3 | 5 | 0.423 | 2 | 5 | 0.216 |
| Anterior chamber inflammation† | 1.33 ± 0.13 | 1.44 ± 0.14 | 0.569 | 1.27 ± 0.13 | 1.30 ± 0.12 | 0.897 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download