초록
Purpose:
To evaluate the clinical efficacy of topical diquafosol tetrasodium 3% ophthalmic solution in patients with short tear film break-up time (BUT) dry eye.
Methods:
This prospective study involved 30 eyes in 30 patients with dry eye who had tear film BUT values ≤5 seconds and schirmer’s 1 test ≥ 5 mm, and showed no improvement with non-preservative sodium hyaluronate (SH) 0.1% artificial tears. All patients were treated with topical diquafosol tetrasodium 3% 6 times a day, in addition to SH 0.1% artificial tears. Schirmer’s 1 test, tear film BUT, keratoepitheliopathy score with fluorescein, conjunctival staining score with lissamine green, and Ocular surface disease index (OSDI) score were evaluated at before treatments, and 1 month and 3 months after treatments.
Results:
Significant improvements of tear film BUT and OSDI were observed at 1 month and 3months after diquafosol tetrasodium 3% administration. At before treatment, and followed up at 1 and 3 months, tear film BUTs were 3.3 ± 1.2, 4.4 ± 1.0 ( p < 0.01) and 4.9 ± 1.1 seconds ( p < 0.01), respectively, and OSDI scores were 43.5 ± 24.4, 34.6 ± 25.0 ( p = 0.01) and 26.7 ± 21.5 ( p < 0.01), respectively. There were no significant changes of Schirmer’s score, keratoepitheliopathy, and conjunctival staining score. After diquafosol tetrasodium 3% administration, severe adverse effects were not found in any of the patients.
Go to : 
References
1. Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003; 136:318–26.

2. Brewitt H, Sistani F. Dry eye disease: the scale of the problem. Surv Ophthalmol. 2001; 45(Suppl 2):S199–202.
3. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5:75–92.
4. Gipson IK, Hori Y, Argüeso P. Character of ocular surface mucins and their alteration in dry eye disease. Ocul Surf. 2004; 2:131–48.

5. Toda I, Fujishima H, Tsubota K. Ocular fatigue is the major symptom of dry eye. Acta Ophthalmol (Copenh). 1993; 71:347–52.

6. Toda I, Shimazaki J, Tsubota K. Dry eye with only decreased tear break-up time is sometimes associated with allergic conjunctivitis. Ophthalmology. 1995; 102:302–9.

7. Fujihara T, Murakami T, Nagano T, et al. INS365 suppresses loss of corneal epithelial integrity by secretion of mucin-like glyco-protein in a rabbit short-term dry eye model. J Ocul Pharmacol Ther. 2002; 18:363–70.

8. Fujihara T, Murakami T, Fujita H, et al. Improvement of corneal barrier function by the P2Y(2) agonist INS365 in a rat dry eye model. Invest Ophthalmol Vis Sci. 2001; 42:96–100.
9. Matsumoto Y, Ohashi Y, Watanabe H, Tsubota K; Diquafosol Ophthalmic Solution Phase 2 Study Group. Efficacy and safety of diquafosol ophthalmic solution in patients with dry eye syndrome: a Japanese phase 2 clinical trial. Ophthalmology. 2012; 119:1954–60.

10. Tauber J, Davitt WF, Bokosky JE, et al. Double-masked, place-bo-controlled safety and efficacy trial of diquafosol tetrasodium (INS365) ophthalmic solution for the treatment of dry eye. Cornea. 2004; 23:784–92.

11. Kamiya K, Nakanishi M, Ishii R, et al. Clinical evaluation of the additive effect of diquafosol tetrasodium on sodium hyaluronate monotherapy in patients with dry eye syndrome: a prospective, randomized, multicenter study. Eye (Lond). 2012; 26:1363–8.

12. Takamura E, Tsubota K, Watanabe H, Ohashi Y; Diquafosol Ophthalmic Solution Phase 3 Study Group. A randomised, dou-ble-masked comparison study of diquafosol versus sodium hyaluronate ophthalmic solutions in dry eye patients. Br J Ophthalmol. 2012; 96:1310–5.

13. Koh S, Ikeda C, Takai Y, et al. Long-term results of treatment with diquafosol ophthalmic solution for aqueous-deficient dry eye. Jpn J Ophthalmol. 2013; 57:440–6.

14. Shimazaki-Den S, Iseda H, Dogru M, Shimazaki J. Effects of diquafosol sodium eye drops on tear film stability in short BUT type of dry eye. Cornea. 2013; 32:1120–5.

15. Kaido M, Uchino M, Kojima T, et al. Effects of diquafosol tetrasodium administration on visual function in short break-up time dry eye. J Ocul Pharmacol Ther. 2013; 29:595–603.

16. Arita R, Suehiro J, Haraguchi T, et al. Topical diquafosol for patients with obstructive meibomian gland dysfunction. Br J Ophthalmol. 2013; 97:725–9.

17. Yoon KC, Heo H, Im SK, et al. Comparison of autologous serum and umbilical cord serum eye drops for dry eye syndrome. Am J Ophthalmol. 2007; 144:86–92.

19. Jeong IY, Park YW, Lee SS, et al. Long term follow-up results of topical 0.05% cyclosporine a in patient with dry eye. Chonnam Med J. 2008; 44:151–6.

20. Dogru M, Katakami C, Inoue M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology. 2001; 108:586–92.

21. Yoon KC, Im SK, Kim HG, You IC. Usefulness of double vital staining with 1% fluorescein and 1% lissamine green in patients with dry eye syndrome. Cornea. 2011; 30:972–6.

22. Kaido M, Goto E, Dogru M, Tsubota K. Punctal occlusion in the management of chronic Stevens-Johnson syndrome. Ophthalmology. 2004; 111:895–900.

23. Yoon KC, Jeong IY, Im SK, et al. Therapeutic effect of umbilical cord serum eyedrops for the treatment of dry eye associated with graft-versus-host disease. Bone Marrow Transplant. 2007; 39:231–5.

24. Yoon KC, Im SK, Park YG, et al. Application of umbilical cord serum eyedrops for the treatment of dry eye syndrome. Cornea. 2006; 25:268–72.

25. Yoon KC, Park CS, You IC, et al. Expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Invest Ophthalmol Vis Sci. 2010; 51:643–50.

26. Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003; 22:640–50.

27. Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000; 118:615–21.

28. Miller KL, Walt JG, Mink DR, et al. Minimal clinically important difference for the ocular surface disease index. Arch Ophthalmol. 2010; 128:94–101.

29. Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf. 2003; 1:107–26.

30. Tsubota K, Nakamori K. Effects of ocular surface area and blink rate on tear dynamics. Arch Ophthalmol. 1995; 113:155–8.

31. Fahmy AM, Hardten DR. Treating ocular surface disease: new agents in development. Clin Ophthalmol. 2011; 5:465–72.
32. Nakamura M, Imanaka T, Sakamoto A. Diquafosol ophthalmic solution for dry eye treatment. Adv Ther. 2012; 29:579–89.

33. Takaoka-Shichijo Y, Sakamoto A, Nakamura M. Effect of diquafosol tetrasodium on MUC5AC secretion by rabbit conjunctival tissues. J Eye. 2011; 28:261–5.
34. Takaoka-Shichijo Y, Nakamura M. Stimulatory effect of diquafosol tetrasodium on the expression of membrane-binding mucin genes in cultured human corneal epithelial cells. J Eye. 2011; 28:425–9.
35. Lombardo M, Lombardo G. Wave aberration of human eyes and new descriptors of image optical quality and visual performance. J Cataract Refract Surg. 2010; 36:313–31.

Go to : 
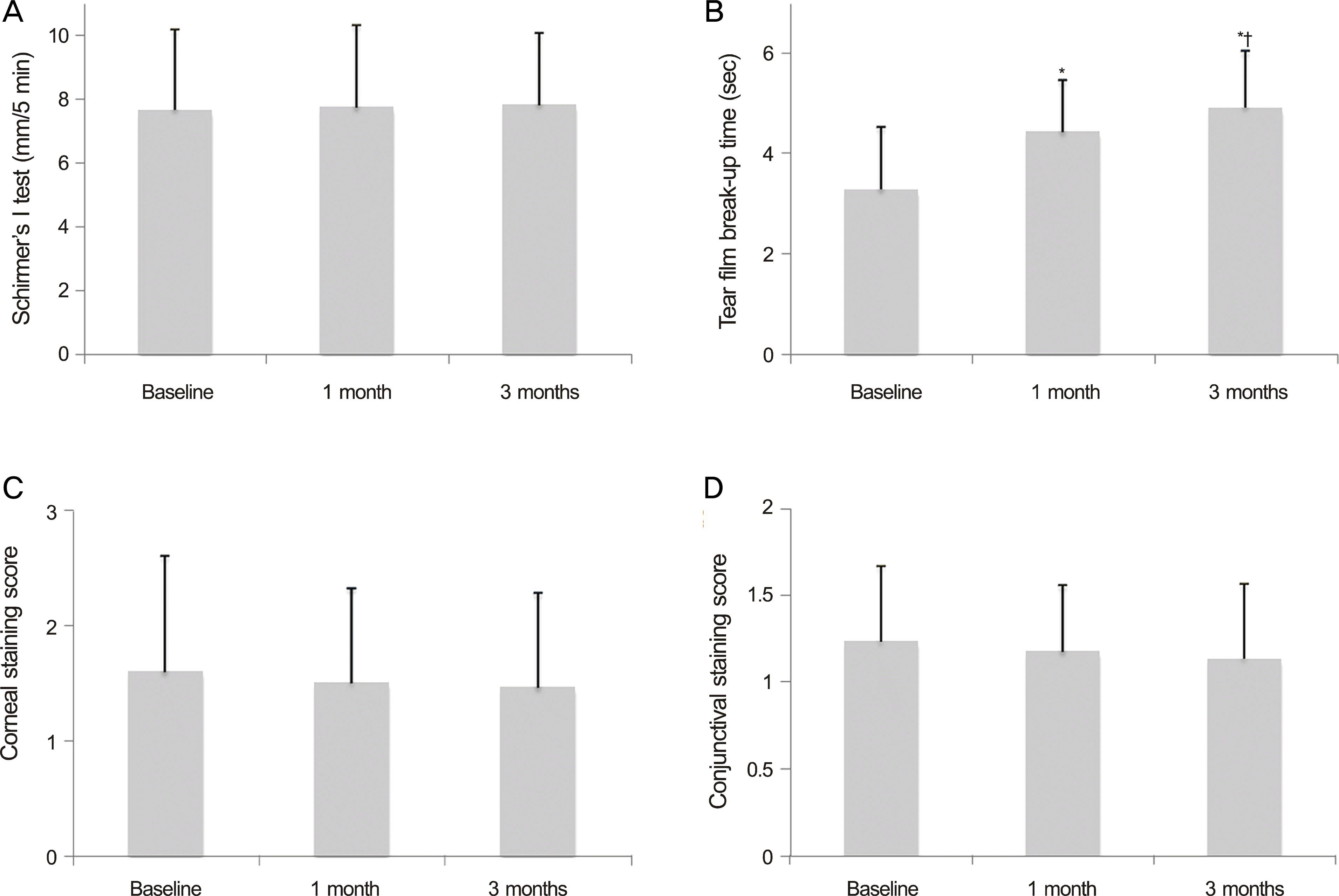 | Figure 1.Changes of tear film and ocular surface parameters before and after treatment with topical 3% diquafosol tetrasodium. (A) Schirmer’s I test. (B) Tear film break-up time. (C) Corneal staining score. (D) Conjunctival staining score. * p < 0.05 compared with baseline; † p < 0.05 compared with 1 month after treatment. |
Table 1.
Demographics of patients with short tear film break-up time dry eye
Table 2.
Changes of ocular surface disease index before and after treatment with topical 3% diquafosol tetrasodium
| OSDI score | Baseline | After 1 month | After 3 months | p-value* | p-value† | p-value‡ |
|---|---|---|---|---|---|---|
| Ocular symptoms (3 items) | 13.2 ± 4.2 | 9.2 ± 5.8 | 5.9 ± 3.9 | <0.01 | <0.01 | <0.01 |
| Vision-related function (6 items) | 20.2 ± 10.7 | 16.4 ± 11.4 | 14.0 ± 8.8 | 0.09 | 0.01 | 0.13 |
| Environmental triggers (3 items) | 10.1 ± 4.7 | 9.0 ± 5.7 | 6.8 ±4.7 | 0.26 | <0.01 | 0.07 |
| Overall | 43.5 ± 24.4 | 34.6 ± 25.0 | 26.7 ± 21.5 | 0.01 | <0.01 | 0.01 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download