Abstract
Purpose
To compare changes in choroidal hyperpermeability after half-energy photodynamic therapy (PDT) and intravitreal ranibizumab in the treatment of chronic central serous chorioretinopathy (CSC).
Methods
Post-hoc analysis was performed in a randomized, controlled trial comparing half-energy PDT versus intravitreal ranibizumab for chronic CSC; during the experiments, the other treatment was available for salvage treatment if the original was un-successful at 3 months. A commercially available image analysis program (Adobe® Photoshop® CS6 [Adobe Systems, Inc., San Jose, CA]) was used for quantification of change in choriodal hyperpermeability on indocyanine green angiography after half-en-ergy PDT or three consecutive intravitreal injections of ranibizumab. Post-treatment images were subtracted from pre-treatment images after adjustments were made to create images depicting the change in choroidal hyperpermeability with treatment. Integrated gray scale values per area in this image were used for analysis of change in choroidal hyperpermeability.
Results
The calculated change in choroidal hyperpermeability was significantly greater in the half-energy PDT group (17.36 ±8.74) than in the ranibizumab group (6.78 ± 5.03) (p < 0.001). All eyes in the half-energy PDT group showed complete resolution of subretinal fluid, and no significant difference in change of choroidal hyperpermeability was found in eyes that received half-en-ergy PDT as primary or salvage treatment. In the ranibizumab-treated group, subretinal fluid resolution was accomplished in 5 eyes, and these eyes showed a significantly larger decrease in choroidal hyperpermeability when compared with eyes showing poor response (10.31 ± 4.00 vs. 2.74 ± 2.16, p = 0.005). In the successfully treated eyes with ranibizumab, there was no significant difference in choroidal hypopermeability change when compared to half-energy PDT (p = 0.124).
Conclusions
Using our novel method of analysis of change in choroidal hyperpermeability following treatment for chronic CSC, greater change was found in eyes with good response, and the superior outcome of half-energy PDT over ranibizumab may be attributed to greater influence on choroidal hyperpermeability.
Go to : 
References
1. Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol. 1967; 63(Suppl):1–139.
2. Spaide RF, Campeas L, Haas A. . Central serous chorioretinop-athy in younger and older adults. Ophthalmology. 1996; 103:2070–9. discussion 2079-80.

3. Jalkh AE, Jabbour N, Avila MP. . Retinal pigment epithelium decompensation. I. Clinical features and natural course. Ophthalmology. 1984; 91:1544–8.
4. Ficker L, Vafidis G, While A, Leaver P. Long-term follow-up of a prospective trial of argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol. 1988; 72:829–34.

5. Iida T, Yannuzzi LA, Spaide RF. . Cystoid macular degeneration in chronic central serous chorioretinopathy. Retina. 2003; 23:1–7. quiz 137-8.

6. Prünte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996; 121:26–34.
7. Spaide RF, Hall L, Haas A. . Indocyanine green videoangiography of older patients with central serous chorioretinopathy. Retina. 1996; 16:203–13.

8. Schaal KB, Hoeh AE, Scheuerle A. . Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. Eur J Ophthalmol. 2009; 19:613–7.

9. Artunay O, Yuzbasioglu E, Rasier R. . Intravitreal bev-acizumab in treatment of idiopathic persistent central serous cho-rioretinopathy: a prospective, controlled clinical study. Curr Eye Res. 2010; 35:91–8.

10. Seong HK, Bae JH, Kim ES. . Intravitreal bevacizumab to treat acute central serous chorioretinopathy: short-term effect. Ophthal- mologica. 2009; 223:343–7.

11. Eandi CM, Ober M, Iranmanesh R. . Acute central serous cho-rioretinopathy and fundus autofluorescence. Retina. 2005; 25:989–93.

12. Schlötzer-Schrehardt U, Viestenz A, Naumann GO. . Dose-re-lated structural effects of photodynamic therapy on choroidal and retinal structures of human eyes. Graefes Arch Clin Exp Ophthalmol. 2002; 240:748–57.

13. Shin JY, Woo SJ, Yu HG, Park KH. Comparison of efficacy and safety between half-fluence and full-fluence photodynamic therapy for chronic central serous chorioretinopathy. Retina. 2011; 31:119–26.

14. Bae SH, Heo JW, Kim C. . A randomized pilot study of lowfluence photodynamic therapy versus intravitreal ranibizumab for chronic central serous chorioretinopathy. Am J Ophthalmol. 2011; 152:784–92.e2.

15. Colucciello M. Choroidal neovascularization complicating photo-dynamic therapy for central serous retinopathy. Retina. 2006; 26:239–42.

16. Chan WM, Lai TY, Lai RY. . Safety enhanced photodynamic therapy for chronic central serous chorioretinopathy: one-year results of a prospective study. Retina. 2008; 28:85–93.
17. Senturk F, Karacorlu M, Ozdemir H. . Microperimetric changes after photodynamic therapy for central serous chorioretinopathy. Am J Ophthalmol. 2011; 151:303–9.e1.

18. Michels S, Hansmann F, Geitzenauer W, Schmidt-Erfurth U. Influence of treatment parameters on selectivity of verteporfin therapy. Invest Ophthalmol Vis Sci. 2006; 47:371–6.

19. Reibaldi M, Cardascia N, Longo A. . Standard-fluence versus low-fluence photodynamic therapy in chronic central serous cho-rioretinopathy: a nonrandomized clinical trial. Am J Ophthalmol. 2010; 149:307–15.e2.

20. Torres-Soriano ME, García-Aguirre G, Kon-Jara V. . A pilot study of intravitreal bevacizumab for the treatment of central serous chorioretinopathy (case reports). Graefes Arch Clin Exp Ophthalmol. 2008; 246:1235–9.

21. Lim SJ, Roh MI, Kwon OW. Intravitreal bevacizumab injection for central serous chorioretinopathy. Retina. 2010; 30:100–6.

22. Carvalho FB, Gonçalves M, Tanomaru-Filho M. Evaluation of chronic periapical lesions by digital subtraction radiography by using Adobe Photoshop CS: a technical report. J Endod. 2007; 33:493–7.

23. Camps J, Pommel L, Bukiet F. Evaluation of periapical lesion healing by correction of gray values. J Endod. 2004; 30:762–6.

24. Angerame D, De Biasi M, Sossi D. . Periapical healing after simplified endodontic treatments: A digital subtraction radiog-raphy study. Giornale Italiano di Endodonzia. 2013; 27:74–9.

25. Queiroz CS, Sarmento VA, de Azevedo RA. . A comparative study of internal fixation and intermaxillary fixation on bone repair of mandibular fractures through radiographic subtraction. J Craniomaxillofac Surg. 2014; 42:e152–6.

26. Costa RA, Farah ME, Freymüller E. . Choriocapillaris photo-dynamic therapy using indocyanine green. Am J Ophthalmol. 2001; 132:557–65.

27. Costa RA, Scapucin L, Moraes NS. . Indocyanine green-medi-ated photothrombosis as a new technique of treatment for persistent central serous chorioretinopathy. Curr Eye Res. 2002; 25:287–97.

28. Guyer DR, Yannuzzi LA, Slakter JS. . Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994; 112:1057–62.

29. Yannuzzi LA, Slakter JS, Gross NE. . Indocyanine green an-giography-guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: a pilot study. Retina. 2003; 23:288–98.
30. Chan WM, Lam DS, Lai TY. . Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol. 2003; 87:1453–8.

Go to : 
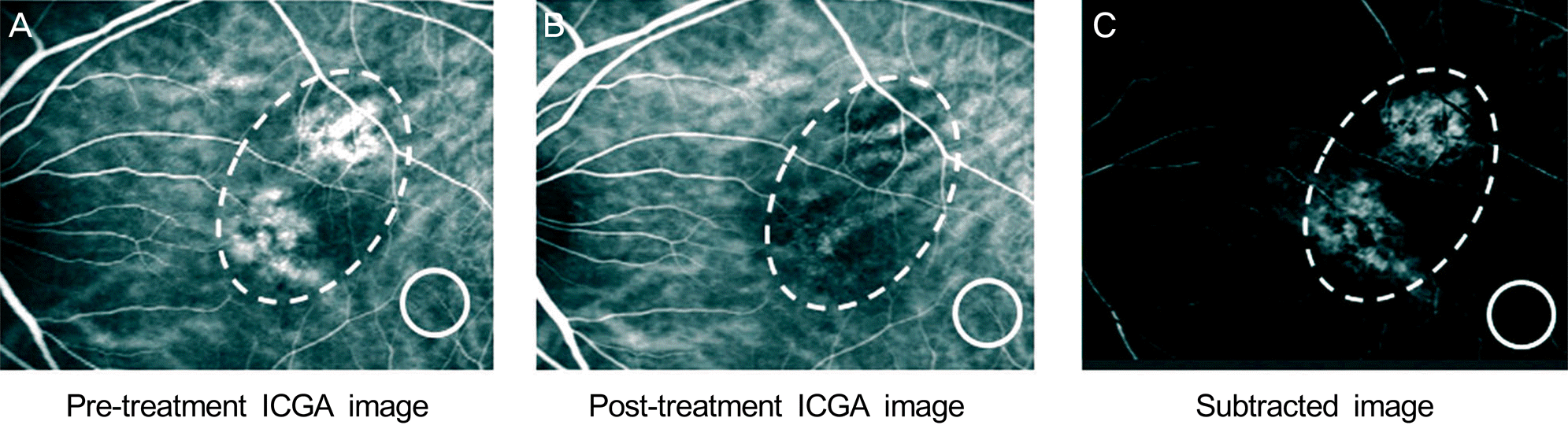 | Figure 1.The method of calculating choroidal hyperpermeability changes after treatment of chronic central serous chorioretinopathy. A 47-year-old female treated with half energy photodynamic therapy (HE-PDT) for chronic central serous chorioretinopathy (CSC). Hyperfluorescent dilated choroidal vessels seen before treatment (A) was disappeared after half-energy PDT (B). The dashed ovoid lines (A, B, and C) indicate the area with choroidal hypermeabity at pretreatment. We quantified the change in choroidal hyper-permeability after treatment for chronic central serous chorioretinopathy using commercially available image analysis software (Adobe® Photoshop® CS6 [Adobe Systems, Inc., San Jose, CA]). By this software, we calculated the mean gray scale in the reference area (circled area in A, B, and C) for correction the contrast difference between pretreatment indocyanine green angiography (ICGA) image and post-treatment ICGA image. We subtracted post-treatment ICGA image from pre-treatment ICGA image to make the subtracted image representing the change in choroidal hyperpermeability after treatment for chronic CSC (C). We calculate in-tegrated gray scale value per area in subtracted image for analysis of change in choroidal hyperpermeability. |
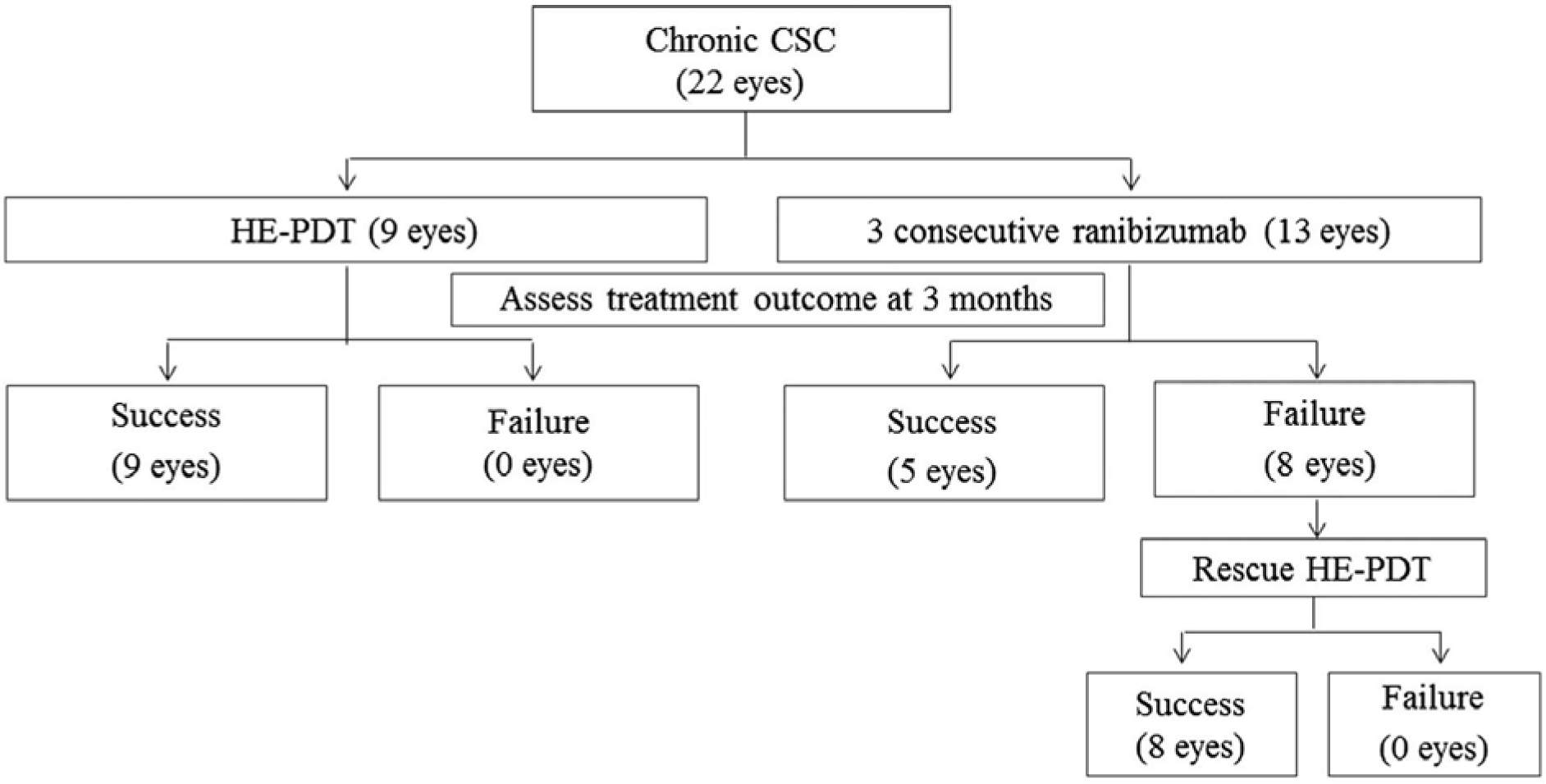 | Figure 2.Flow chart of treatment progress. CSC = central serous chorioretinopathy; HE-PDT = half energy photo-dynamic therapy. |
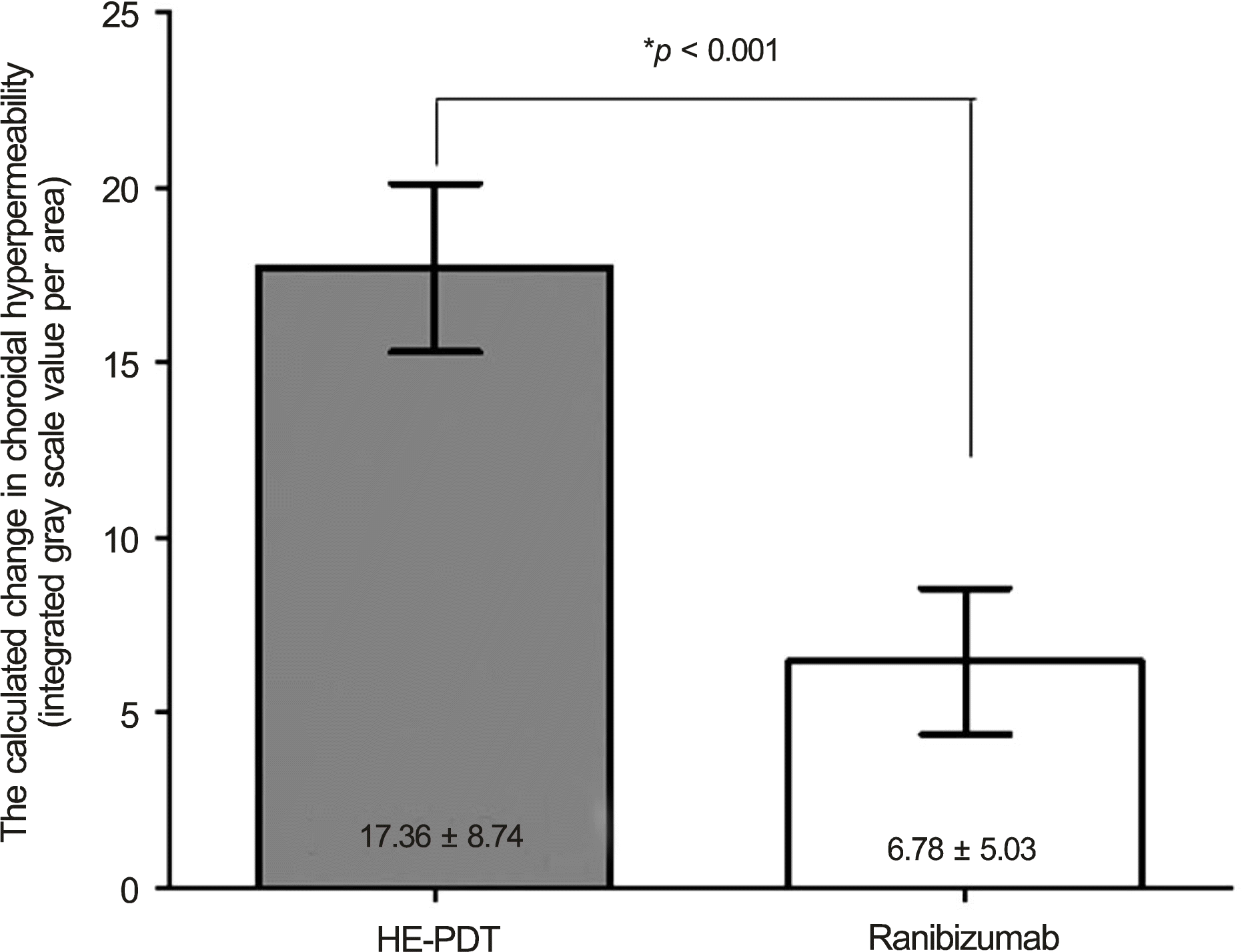 | Figure 3.The calculated changes in choroidal hyperpermeability after treatment for chronic central serous chorioretinopathy according to primary treatment methods (HE-PDT versus 3 consecutive intravitreal ranibizumab injections). HE-PDT = half * Between-group comparison energy photodynamic therapy. was evaluated by Mann-Whitney U-test. |
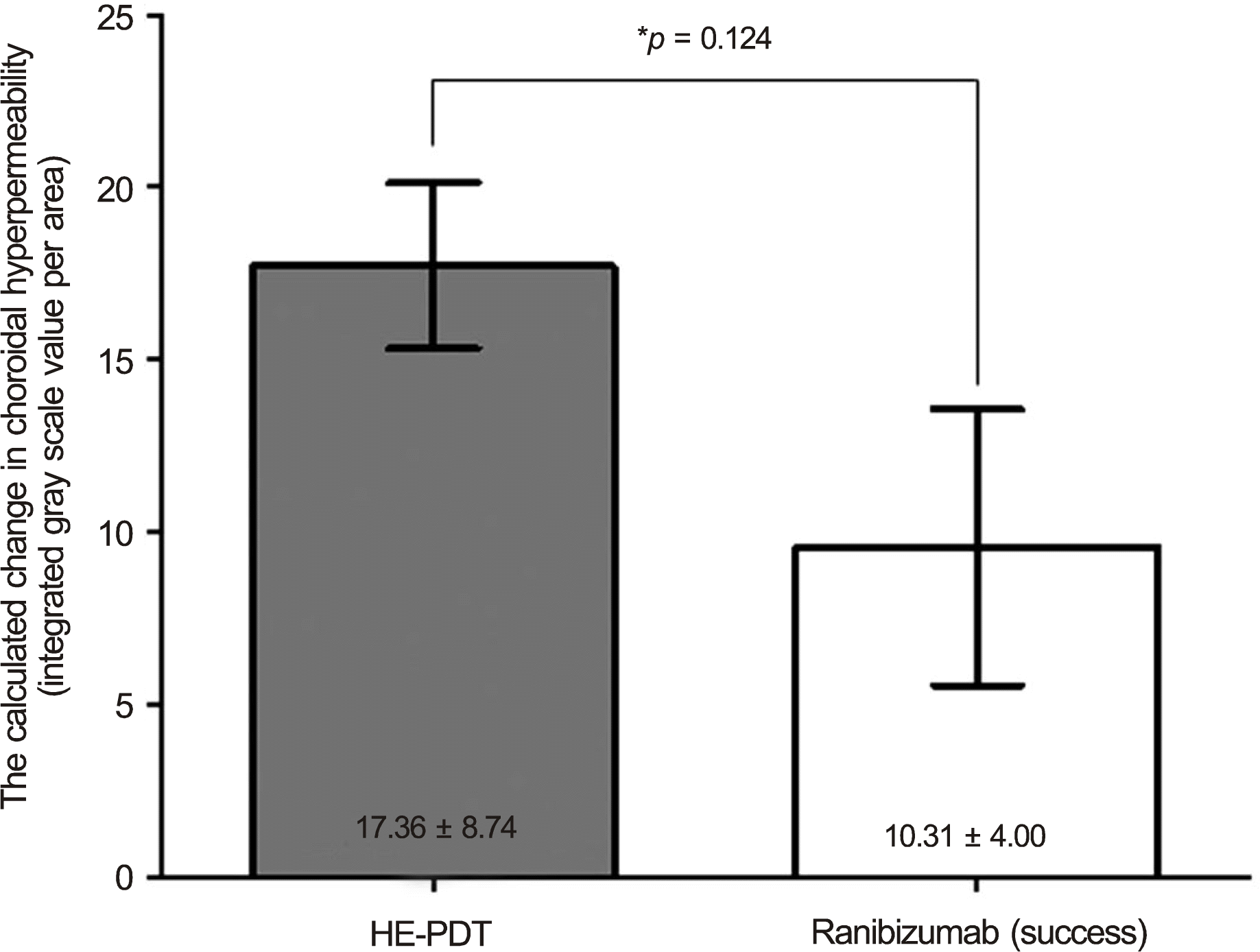 | Figure 4.The comparison of change of choroidal hyper-permeability after treatment in the successfully treated eyes for chronic central serous chorioretinopathy (HE-PDT versus 3 consecutive intravitreal ranibizumab injections). HE-PDT = half energy photodynamic therapy. * Between-group comparison was evaluated by Mann-Whitney U-test. |
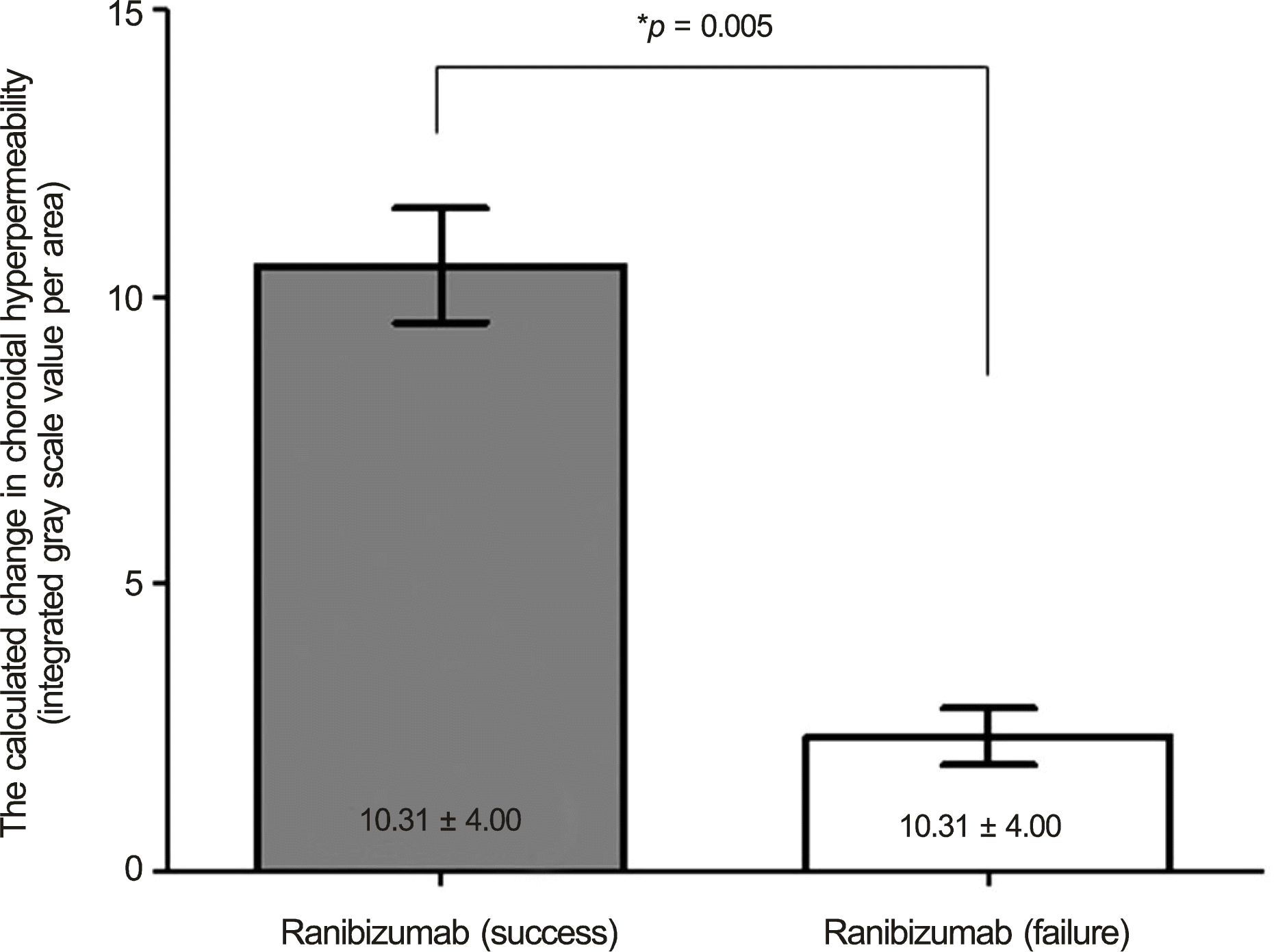 | Figure 5.The comparison of change of choroidal hyper-permeability after treatment in the eyes treated with 3 consecutive intravitreal ranibizumab injections for chronic central serous chorioretinopathy. * Between-group comparison was evaluated by Mann-Whitney U-test. |
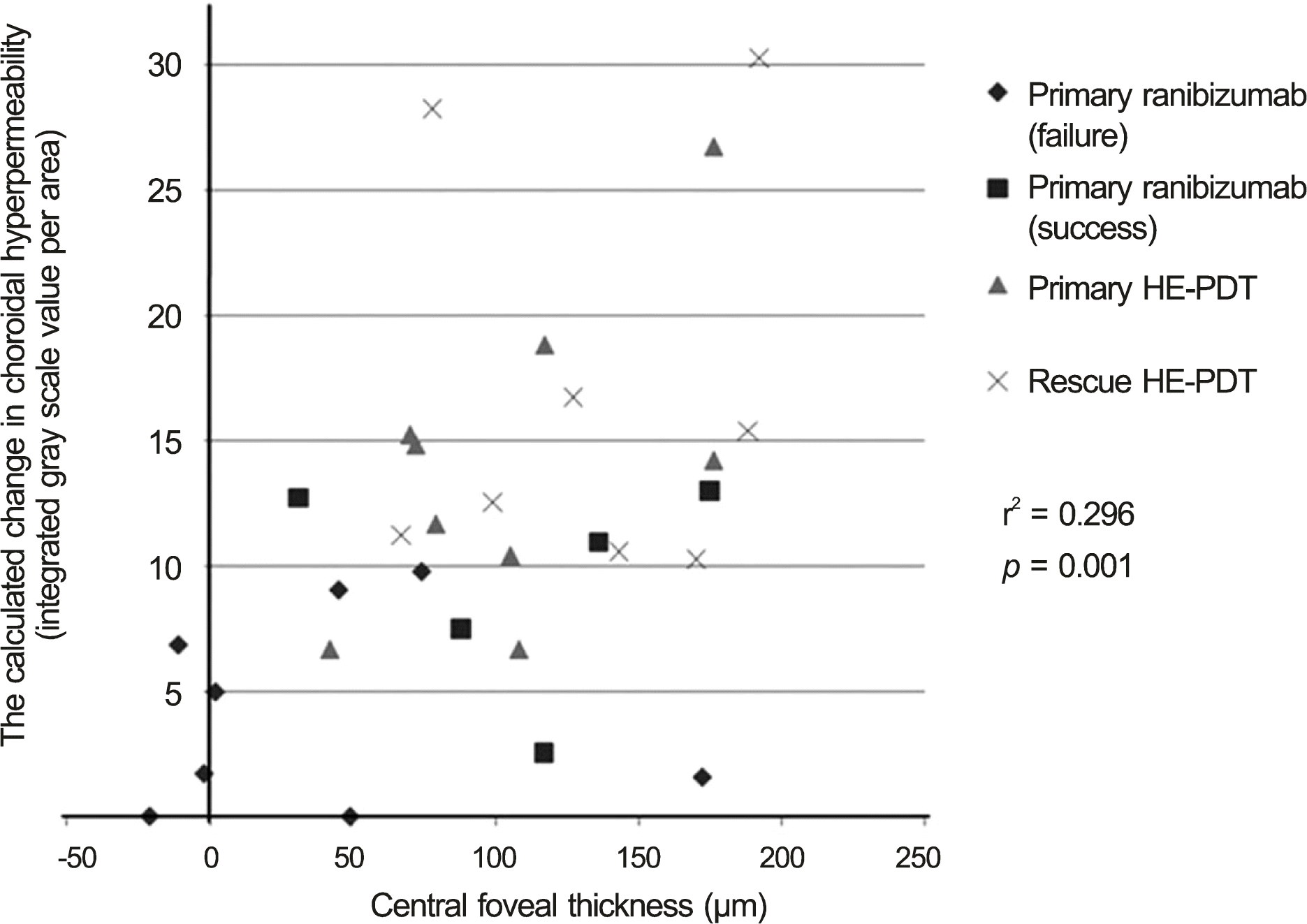 | Figure 6.The association of the change in choroidal hyper-permeability with decrease in central forveal thickness. HE-PDT = half energy photodynamic therapy. |
Table 1.
Demographic characteristics in each group
| Baseline characteristics | Half-energy PDT (n = 9) | Ranibizumab (n = 13) | p-value |
|---|---|---|---|
| Sex (M:F) | 8:1 | 12:1 | |
| Age (years) | 52.89 | 51.80 | 0.929‡ |
| BCVA | 0.36 ± 0.19 | 0.35 ± 0.15 | 0.846‡ |
| CFT (μ m) | 303.2 ± 43.9 | 297.2 ± 61.4 | 0.421‡ |
| Choroidal hyperpermeability with dilated choroidal vasculature | 9 (100) | 13 (100) | 0.999 |
| on ICGA (n, %) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download