Abstract
Purpose
To evaluate the short-term effect of intravitreal aflibercept (Eylea®; Regeneron Pharmaceuticals Inc., Tarrytown, NY, USA and Bayer, Basel, Switzerland) on the visual outcomes and retinal anatomic changes of patients with polypoidal choroidal vasculopathy (PCV).
Methods
Intravitreal Eylea® was injected into 16 eyes of 16 patients with PCV in this retrospective case study. After therapy, the patients were followed up for over 3 months. Changes in best-corrected visual acuity (BCVA) and central foveal thickness (CFT) using optical coherence tomography (OCT) and abnormal vasculature on indocyanine green angiography (ICGA) were evaluated.
Results
The mean log MAR BCVA was 0.75 ± 0.60 at baseline, 0.74 ± 0.60 and 0.71 ± 0.63 at 1 and 2 months, respectively ( p > 0.05) and 0.57 ± 0.53 at 3 months (p < 0.05) after treatment. The mean CFT was 379 ± 130 μm at baseline, 281 ± 92 μm, 247 ± 54 μ m, and 231 ± 51 μ m at 1, 2, and 3 months, respectively, after treatment ( p < 0.05). Complete resolution was 43%, 55%, and 50% at 1, 2, and 3 months, respectively in pigment epithelial detachment (PED), 67%, 83%, and 92% at 1, 2, and 3 months, re-spectively in subretinal fluid (SRF) and 33%, 60%, and 60% at 1, 2, and 3 months, respectively in intraretinal fluid (IRF) using OCT. The polypoidal lesions in ICGA decreased in 12 of 14 eyes (86%).
References
1. Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic poly-poidal choroidal vasculopathy (IPCV). Retina. 1990; 10:1–8.

2. Ciardella AP, Donsoff IM, Huang SJ. . Polypoidal choroidal vasculopathy. Surv Ophthalmol. 2004; 49:25–37.

3. Uyama M, Matsubara T, Fukushima I. . Idiopathic polypoidal choroidal vasculopathy in Japanese patients. Arch Ophthalmol. 1999; 117:1035–42.

4. Uyama M, Wada M, Nagai Y. . Polypoidal choroidal vasculop-athy: natural history. Am J Ophthalmol. 2002; 133:639–48.
5. Lee WK, Kwon SI. Polypoidal choroidal vasculopathy. J Korean Ophthalmol Soc. 2000; 41:2573–84.
6. Scassellati-Sforzolini B, Mariotti C, Bryan R. . Polypoidal choroidal vasculopathy in Italy. Retina. 2001; 21:121–5.

7. Lafaut BA, Leys AM, Snyers B. . Polypoidal choroidal vascul-opathy in Caucasians. Graefes Arch Clin Exp Ophthalmol 2000; 238. 752–9.

8. Yannuzzi LA, Wong DW, Sforzolini BS. . Polypoidal choroi-dal vasculopathy and neovascularized age-related macular degen-eration. Arch Ophthalmol. 1999; 117:1503–10.

9. Sho K, Takahashi K, Yamada H. . Polypoidal choroidal vascul-opathy: incidence, demographic features, and clinical characte-ristics. Arch Ophthalmol. 2003; 121:1392–6.
10. Wen F, Chen C, Wu D, Li H. Polypoidal choroidal vasculopathy in elderly Chinese patients. Graefes Arch Clin Exp Ophthalmol. 2004; 242:625–9.

11. Byeon SH, Lee SC, Oh HS. . Incidence and clinical patterns of polypoidal choroidal vasculopathy in Korean patients. Jpn J Ophthalmol. 2008; 52:57–62.

12. Rosa RH Jr, Davis JL, Eifrig CW. Clinicopathologic reports, case reports, and small case series: clinicopathologic correlation of idio-pathic polypoidal choroidal vasculopathy. Arch Ophthalmol. 2002; 120:502–8.
13. Kuroiwa S, Tateiwa H, Hisatomi T. . Pathological features of surgically excised polypoidal choroidal vasculopathy membranes. Clin Experiment Ophthalmol. 2004; 32:297–302.

14. Yuzawa M, Mori R, Kawamura A. The origins of polypoidal cho-roidal vasculopathy. Br J Ophthalmol. 2005; 89:602–7.

15. Tong JP, Chan WM, Liu DT. . Aqueous humor levels of vas-cular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol. 2006; 141:456–62.

16. Matsuoka M, Ogata N, Otsuji T. . Expression of pigment epi-thelium derived factor and vascular endothelial growth factor in choroidal neovascular membranes and polypoidal choroidal vasculopathy. Br J Ophthalmol. 2004; 88:809–15.

17. Cho HJ, Kim JW, Lee DW. . Intravitreal bevacizumab and ranibizumab injections for patients with polypoidal choroidal vasculopathy. Eye (Lond). 2012; 26:426–33.

18. Cho HJ, Baek JS, Lee DW. . Short-term effectiveness of intravitreal bevacizumab vs. ranibizumab injections for patients with polypoidal choroidal vasculopathy. Korean J Ophthalmol. 2012; 26:157–62.

19. Inoue M, Arakawa A, Yamane S, Kadonosono K. Short-term effi-cacy of intravitreal aflibercept in treatment-naive patients with pol-ypoidal choroidal vasculopathy. Retina. 2014; 34:2178–84.

20. Yuzawa M, Mori R, Haruyama M. A study of laser photocoagul-ation for polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2003; 47:379–84.

21. Lee SC, Seong YS, Kim SS. . Photodynamic therapy with ver-teporfin for polypoidal choroidal vasculopathy of the macula. Ophthalmologica. 2004; 218:193–201.

22. Lee PY, Kim KS, Lee WK. Photodynamic therapy with verteporfin in polypoidal choroidal vasculopathy. J Korean Ophthalmol Soc. 2004; 45:216–27.
23. Silva RM, Figueira J, Cachulo ML. . Polypoidal choroidal vas-culopathy and photodynamic therapy with verteporfin. Graefes Arch Clin Exp Ophthalmol. 2005; 243:973–9.

24. Gomi F, Sawa M, Sakaguchi H. . Efficacy of intravitreal bev-acizumab for polypoidal choroidal vasculopathy. Br J Ophthalmol. 2008; 92:70–3.

25. Kokame GT, Yeung L, Lai JC. Continuous anti-VEGF treatment with ranibizumab for polypoidal choroidal vasculopathy: 6-month results. Br J Ophthalmol. 2010; 94:297–301.

26. Stewart MW, Rosenfeld PJ. Predicted biological activity of intra-vitreal VEGF Trap. Br J Ophthalmol. 2008; 92:667–8.

27. Papadopoulos N, Martin J, Ruan Q. . Binding and neutraliza-tion of vascular endothelial growth factor (VEGF) and related li-gands by VEGF Trap, ranibizumab and bevacizumab. Angiogen-esis. 2012; 15:171–85.

28. Heier JS, Brown DM, Chong V. . Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012; 119:2537–48.

Figure 1.
Change in logarithm of the minimum angle of reso-lution (log MAR), best-corrected visual acuity with time (months). Decrease at 1, 2, and 3 months was observed. A sig-nificant decrease was found at 3 months ( p < 0.05). Case 8, 11 were loss to FU after 1 month. FU = follow-up.
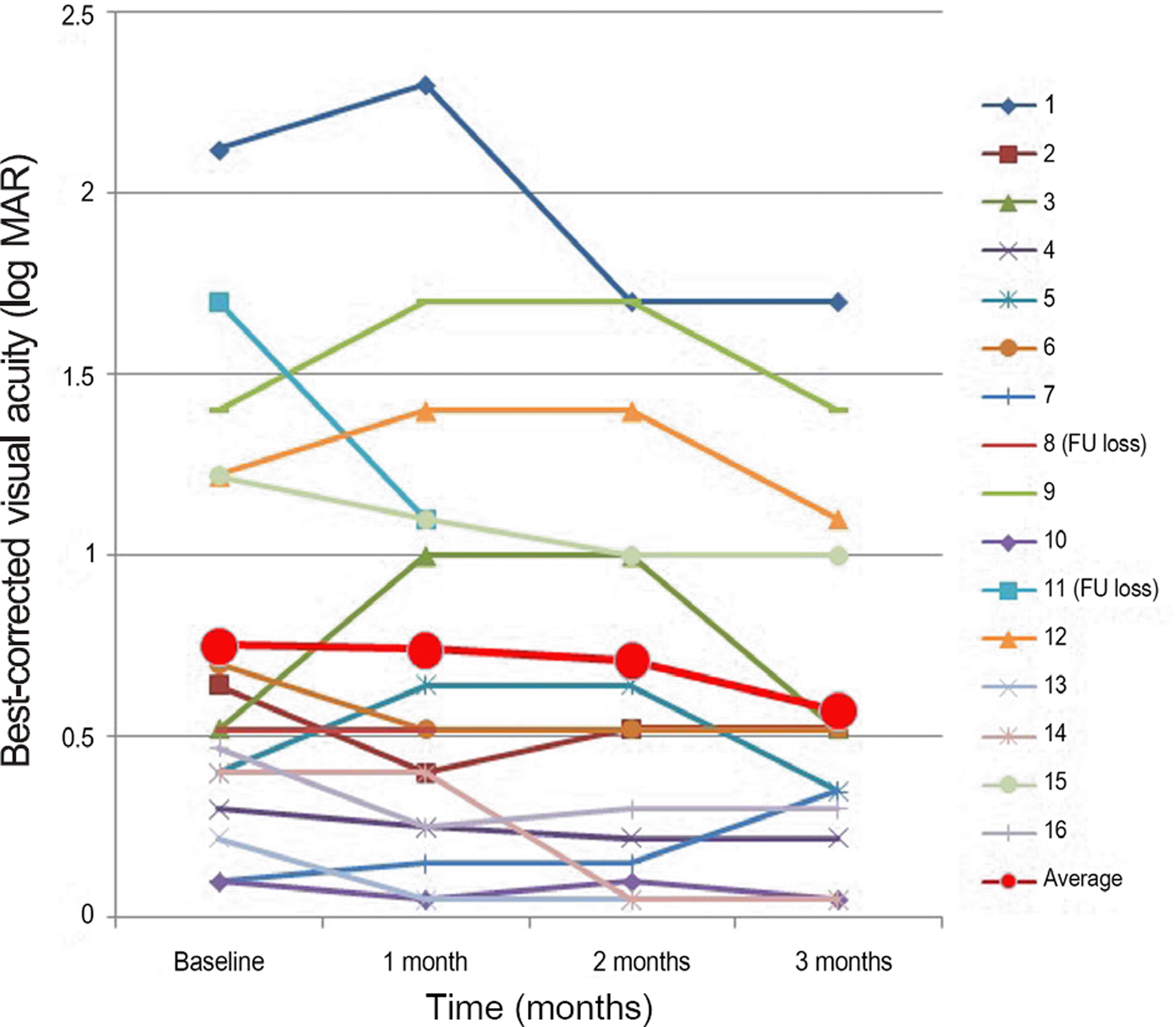
Figure 2.
Change in percentage (%), in proportion of more than 0.2 log MAR with time (months). Increase in proportion of gain more than 0.2 log MAR, decrease in proportion of loss more than 0.2 log MAR with time was observed. log MAR = logarithm of the minimum angle of resolution.
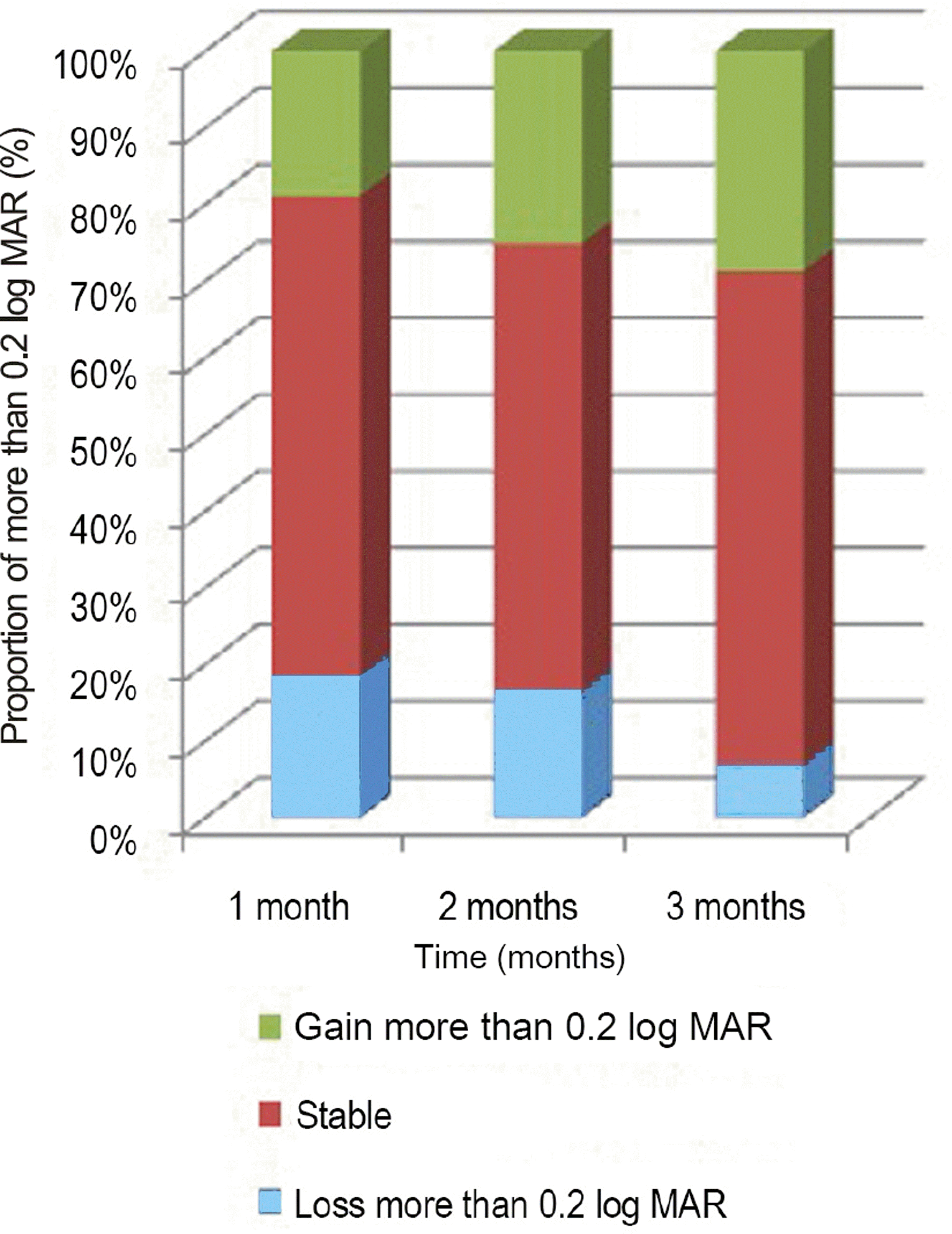
Figure 3.
Change in CFT (μ m) with time (months). A sig-nificant decrease was found at 1, 2, and 3months ( p < 0.05). Case 8, 11 were loss to FU after 1 month. CFT = central fo-veal thickness; FU = follow-up.
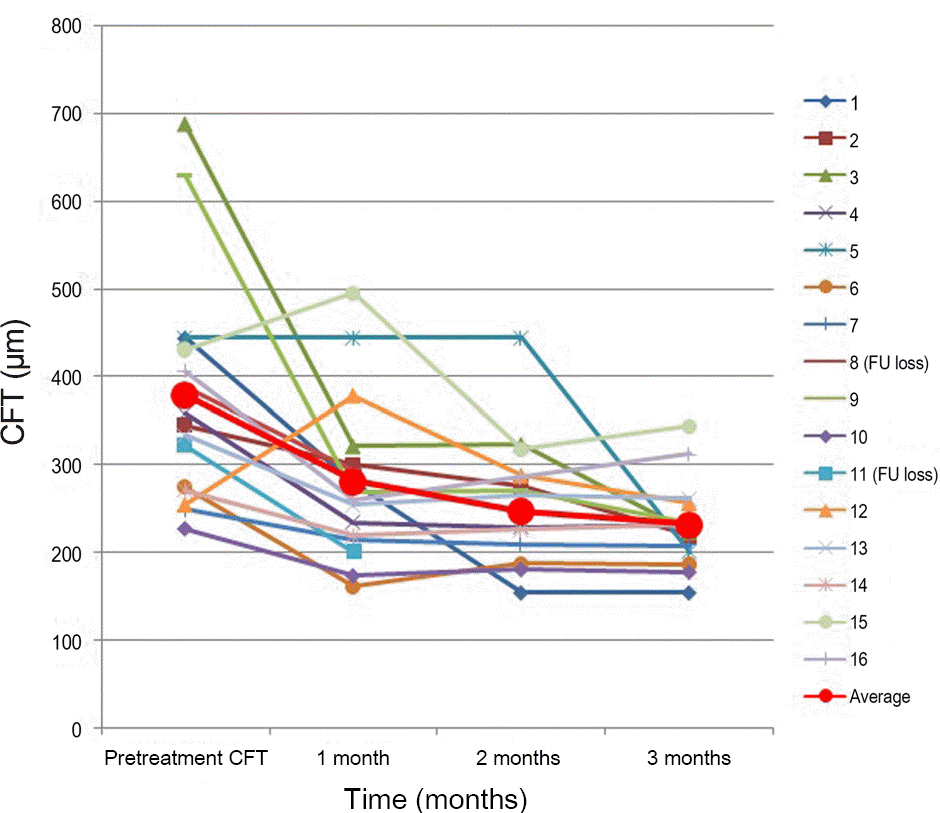
Figure 4.
Case No. 5 (A) Baseline fundus photograph shows hard exudates, pigmentations around macula. (B) Baseline horizontal OCT image and (C) vertical OCT image shows protruding polyps and macular edema. (D-F) One month after the third injection, PED, SRF was completely resolved. OCT = optical coherence tomography; PED = pigment epithelial detachment; SRF= sub-retinal fluid.

Figure 5.
Case No. 5 (A) Baseline ICGA shows polypoidal dilatation of choroidal vessels and branching vascular network. (B) One month after the third injection, polyps are resolved. But branching vascular network remains. ICGA = indocyanine green angiography.

Table 1.
Patient characterstics and clinical data before and after intravitreal injection of aflibercept for PCV
Table 2.
Change in percentage (%), in proportion of partial or complete solution in PED, SRF, IRF by OCT




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download