Abstract
Purpose
To investigate the clinical results of patients who have undergone simultaneous dermofat graft and insertion of orbital implants in patients who are unable to put on an ocular prosthesis due to severe conjunctival sac contracture or large orbital implant exposure.
Methods
A retrospective analysis was performed of patients who underwent dermofat graft simultaneously with orbital implant insertion for replacement of the conjunctival sac from 2007 to 2012. Eight eyes were enrolled in this study and all patients were followed up for phthisis bulbi or implant exposure.
Results
Among the eight eyes, five eyes (62.5%) that were diagnosed with orbital implant exposure underwent orbital implant exchange and dermofat graft, and two eyes (25%) were anophthalmic enophthalmic patients and underwent secondary orbital implant insertion and dermofat graft. One patient (12.5%) underwent orbital implant insertion and dermofat graft simultaneously during the evisceration operation. We followed the progress for 46.3 months.
For seven out of eight eyes, the results of the wound healing process were successful. One patient underwent removal and reinsertion of the orbital implant with dermofat graft, and the wound in this case healed well. However, after five months, dermofat regraft was performed for orbital implant reexposure and it was not exposed thereafter. Overall cosmetic appearance was satisfactory in each patient, and all patients were able to comfortably retain a prosthesis.
Go to : 
References
1. Yoon KC, Ji YS, Park YG. Management of exposed hydroxyapatite implant with acellular dermal allograft. J Korean Ophthalmol Soc. 2005; 46:927–32.
2. Park MS, Kim KS, Baek SH, Lee TS. Management of exposed porous orbital implant with autogenous dermis graft. J Korean Ophthalmol Soc. 2001; 42:1127–32.
3. Sohn KS, Kim JW, Lee TS. Clinical observation on reconstruction of the anophthalmic contracted socket in 28 eyes. J Korean Ophthalmol Soc. 1986; 27:471–4.
4. Gougelmann HP. The evolution of the ocular motility implant. International Ophthalmology Clinics. 1970; 10:689–711.

7. Kostick DA, Linberg JV. Evisceration with hydroxyapatite implant. Surgical technique and review of 31 case reports. Ophthalmology. 1995; 102:1542–8. discussion 1548-9.
8. You YS, Kim HY, Lee SY. Incidence and clinical course of implant exposure after hydroxyapatite implantation in anophthalmic socket. J Korean Ophthalmol Soc. 1997; 38:1694–9.
10. Li T, Shen J, Duffy MT. Exposure rates of wrapped and unwrapped orbital implants following enucleation. Ophthal Plast Reconstr Surg. 2001; 17:431–5.

11. McNab A. Hydroxyapatite orbital implants. Experience with 100 cases. Aust N Z J Ophthalmol. 1995; 23:117–23.

12. Oestreicher JH, Liu E, Berkowitz M. Complications of hydrox-yapatite orbital implants. A review of 100 consecutive cases and a comparison of Dexon mesh (polyglycolic acid) with scleral wrapping. Ophthalmology. 1997; 104:324–9.
13. Custer PL, Trinkaus KM. Porous implant exposure: Incidence, management, and morbidity. Ophthal Plast Reconstr Surg. 2007; 23:1–7.

14. Nunery WR, Heinz GW, Bonnin JM. . Exposure rate of hydroxyapatite spheres in the anophthalmic socket: histopathologic correlation and comparison with silicone sphere implants. Ophthal Plast Reconstr Surg. 1993; 9:96–104.
15. Remulla HD, Rubin PA, Shore JW. . Complications of porous spherical orbital implants. Ophthalmology. 1995; 102:586–93.

16. Yoon JS, Lew H, Kim SJ, Lee SY. Exposure rate of hydroxyapatite orbital implants a 15-year experience of 802 cases. Ophthalmology. 2008; 115:566–72.e2.
17. Baek SH. Clinical effect of porous polyethylene (Medpor(r)) orbital implant. J Korean Ophthalmol Soc. 2000; 41:1858–63.
18. Karcioglu ZA, al-Mesfer SA, Mullaney PB. Porous polyethylene orbital implant in patients with retinoblastoma. Ophthalmology. 1998; 105:1311–6.
19. Rosen HM, McFarland MM. The biologic behavior of hydrox-yapatite implanted into the maxillofacial skeleton. Plast Reconstr Surg. 1990; 85:718–23.

20. Goldberg RA, Holds JB, Ebrahimpour J. Exposed hydroxyapatite orbital implants. Report of six cases. Ophthalmology. 1992; 99:831–6.
21. Kim YD, Goldberg RA, Shorr N, Steinsapir KD. Management of exposed hydroxyapatite orbital implants. Ophthalmology. 1994; 101:1709–15.

22. Lee MJ, Khwarg SI, Choung HK. . Dermis-fat graft for treatment of exposed porous polyethylene implants in pediatric post-enucleation retinoblastoma patients. Am J Ophthalmol. 2011; 152:244–50.e2.

Go to : 
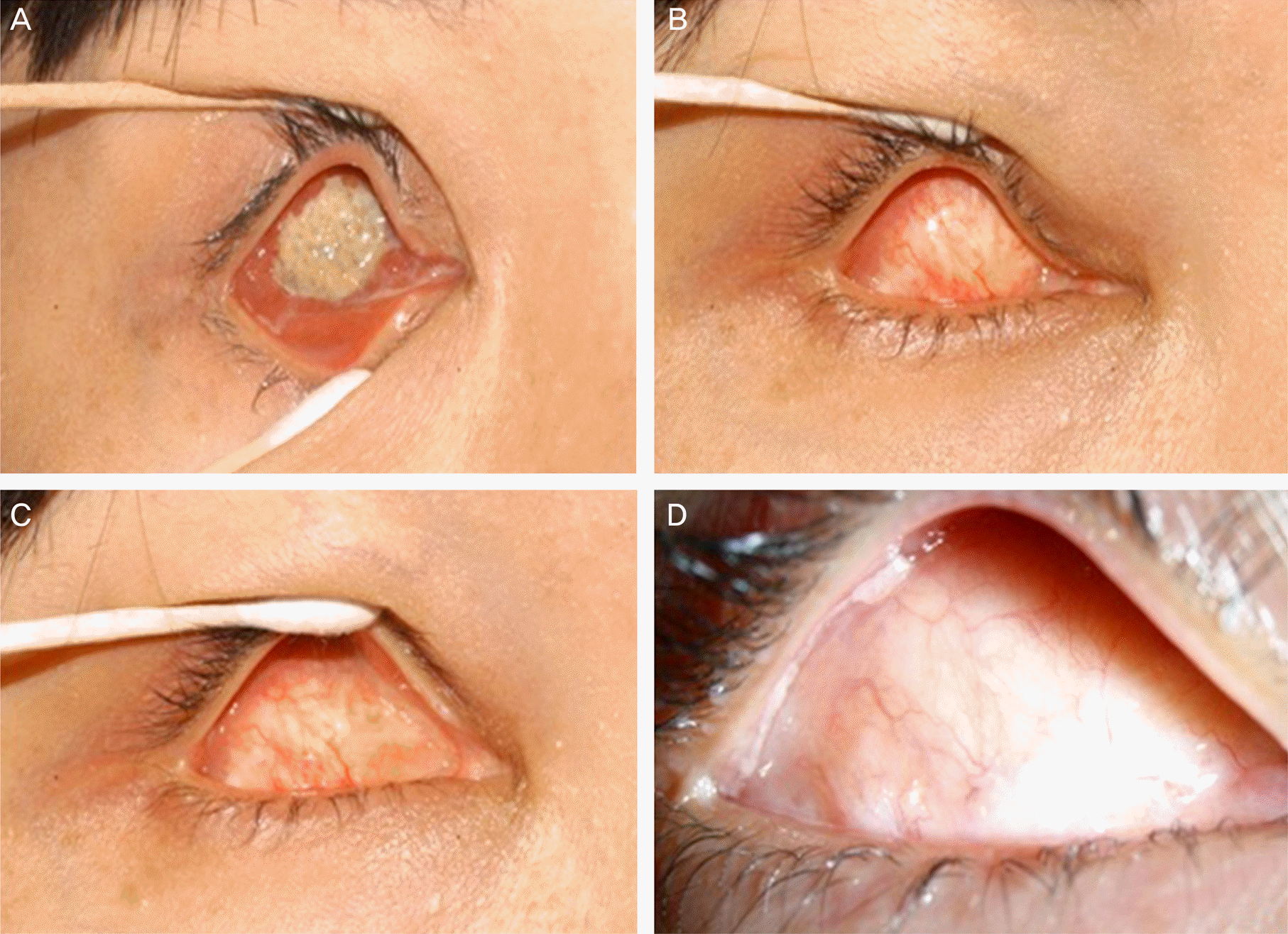 | Figure 1.(A) Preoperative photograph of an exposed orbital implant at a 31-year-old female. (B) Post-operative (6 weeks after dermis fat graft) photograph. (C) Post-operative (1 year after dermis fat graft) photograph. (D) At post-oper-ative 4 years after dermis fat graft, no evidence of reexposure is observed and wound was clear and well conjunctivalization (case 3). |
Table 1.
Demographics and baseline characteristics of patients
| Case | Age/ Sex | Etiology | Surgery | Implant | Implant size (mm) | Exposure size | Donor site | F/U period (months) | State of grafted dermo-fat | Donor site complications |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/72 | Implant | Implant | HA | 18 | Large∗ | Abdomen | 25 | Well-grafted, | None |
| exposure | exchange | no exposure | ||||||||
| 2 | F/66 | Anophthalmos, | Secondary | HA | 18 | - | Abdomen | 32 | Well-grafted, | None |
| enophthalmos | implant | no exposure | ||||||||
| insertion | ||||||||||
| 3 | F/31 | Implant | Implant | Silicone | 20 | Large | Abdomen | 51 | Well-grafted, | None |
| exposure | exchange | ball | no exposure | |||||||
| 4 | F/73 | Implant | Implant | HA | 18 | Large | Abdomen | 55 | Well-grafted, | None |
| exposure | exchange | no exposure | ||||||||
| 5 | F/51 | Anophthalmos, | Secondary | HA | 20 | - | Abdomen | 38 | Well-grafted, | None |
| enophthalmos | implant | no exposure | ||||||||
| insertion | ||||||||||
| 6 | M/61 | Anophthalmos, | Evisceration | HA | 20 | - | Abdomen | 57 | Well-grafted, | None |
| enophthalmos | no exposure | |||||||||
| 7 | M/56 | Implant | Implant | HA | 18 | Large | Abdomen | 36 | Well-grafted, | None |
| exposure | exchange | no exposure | ||||||||
| 8 | M/72 | Implant | Implant | Medpore | 16 | Large | Abdomen | 75 | Exposed of graft | None |
| exposure | exchange | site |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download