Abstract
Purpose
To evaluate the therapeutic effects of 0.03% tacrolimus eye drops on dry eye associated with chronic ocular graft-ver-sus-host disease (GVHD).
Methods
This study included 24 eyes of 12 patients with refractory dry eye associated with chronic ocular GVHD who were un-responsive to topical steroid and 0.05% topical cyclosporine. The topical steroid and cyclosporine were discontinued for 2 weeks before treatment and 0.03% tacrolimus eye drops were applied twice a day for 1 month. Artificial tears, ointment, and lid cleanser were used in the same manner before initiating tacrolimus treatment. Ocular staining score, tear break-up time, ocular surface disease index (OSDI), and Schirmer I test score were evaluated.
References
2. Anderson BE, McNiff JM, Jain D. . Distinct roles for donor- and host-derived antigen-presenting cells and costimulatory mole-cules in murine chronic graft-versus-host disease: requirements de-pend on target organ. Blood. 2005; 105:2227–34.

3. Tabbara KF, Al-Ghamdi A, Al-Mohareb F. . Ocular findings af-ter allogeneic hematopoietic stem cell transplantation. Ophthalmology. 2009; 116:1624–9.

4. Westeneng AC, Hettinga Y, Lokhorst H. . Ocular graft-ver-sus-host disease after allogeneic stem cell transplantation. Cornea. 2010; 29:758–63.

5. Ogawa Y, Okamoto S, Kuwana M. . Successful treatment of dry eye in two patients with chronic graft-versus-host disease with systemic administration of FK506 and corticosteroids. Cornea. 2001; 20:430–4.

6. Tam PM, Young AL, Cheng LL, Lam PT. Topical 0.03% tacroli-mus ointment in the management of ocular surface inflammation in chronic GVHD. Bone Marrow Transplant. 2010; 45:957–8.

7. Ogawa Y, Okamoto S, Mori T. . Autologous serum eye drops for the treatment of severe dry eye in patients with chronic graft-ver-sus-host disease. Bone Marrow Transplant. 2003; 31:579–83.

8. Espana EM, Shah S, Santhiago MR, Singh AD. Graft versus host disease: clinical evaluation, diagnosis and management. Graefes Arch Clin Exp Ophthalmol. 2013; 251:1257–66.

9. Robinson MR, Lee SS, Rubin BI. . Topical corticosteroid ther-apy for cicatricial conjunctivitis associated with chronic graft-ver-sus-host disease. Bone Marrow Transplant. 2004; 33:1031–5.

10. Cheng AC, Yuen K, Chan W. Topical tacrolimus ointment for treatment of refractory anterior segment inflammatory disorders. Cornea. 2006; 25:634. author reply 634.

11. Frandsen E. Glaucoma and cataract as complications to topical ste-roid therapy. Acta Ophthalmol (Copenh). 1966; 44:307–12.

12. Kino T, Hatanaka H, Hashimoto M. . FK-506, a novel im-munosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J Antibiot (Tokyo). 1987; 40:1249–55.

13. Jensik SC. Tacrolimus (FK 506) in kidney transplantation: three- year survival results of the US multicenter, randomized, com-parative trial. FK 506 Kidney Transplant Study Group. Transplant Proc. 1998; 30:1216–8.
14. Griffith BP, Bando K, Hardesty RL. . A prospective random-ized trial of FK506 versus cyclosporine after human pulmonary transplantation. Transplantation. 1994; 57:848–51.
15. Aqel BA, Machicao V, Rosser B. . Efficacy of tacrolimus in the treatment of steroid refractory autoimmune hepatitis. J Clin Gastroenterol. 2004; 38:805–9.

16. Schreiber SL, Crabtree GR. The mechanism of action of cyclo-sporin A and FK506. Immunol Today. 1992; 13:136–42.

17. Fung JJ, Eliasziw M, Todo S. . The Pittsburgh randomized trial of tacrolimus compared to cyclosporine for hepatic transplantation. J Am Coll Surg. 1996; 183:117–25.
18. Lee YJ, Kim SW, Seo KY. Application for tacrolimus ointment in treating refractory inflammatory ocular surface diseases. Am J Ophthalmol. 2013; 155:804–13.

19. Zhai J, Gu J, Yuan J, Chen J. Tacrolimus in the treatment of ocular diseases. BioDrugs. 2011; 25:89–103.

20. Jung JW, Lee YJ, Yoon SC. . Long-term result of maintenance treatment with tacrolimus ointment in chronic ocular graft-ver-sus-host disease. Am J Ophthalmol. 2015; 159:519–27.e1.

21. Ryu EH, Kim JM, Laddha PM. . Therapeutic effect of 0.03% tacrolimus ointment for ocular graft versus host disease and vernal keratoconjunctivitis. Korean J Ophthalmol. 2012; 26:241–7.

22. Miyazaki D, Tominaga T, Kakimaru-Hasegawa A. . Therapeutic effects of tacrolimus ointment for refractory ocular surface in-flammatory diseases. Ophthalmology. 2008; 115:988–92.e5.

23. Moscovici BK, Holzchuh R, Chiacchio BB. . Clinical treat-ment of dry eye using 0.03% tacrolimus eye drops. Cornea. 2012; 31:945–9.

24. Sanz-Marco E, Udaondo P, García-Delpech S. . Treatment of refractory dry eye associated with graft versus host disease with 0.03% tacrolimus eyedrops. J Ocul Pharmacol Ther. 2013; 29:776–83.

25. Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003; 22:640–50.

26. Hyon JY, Kim HM, Lee D. . Korean guidelines for the diag-nosis and management of dry eye: development and validation of clinical efficacy. Korean J Ophthalmol. 2014; 28:197–206.

27. Lee SH, Im SK, Woo JM, Yoon KC. Long-term evaluation after topical cyclosporine treatment in dry eye patients with graft-ver-sus-host disease. J Korean Ophthalmol Soc. 2009; 50:27–33.

28. Lelli GJ Jr, Musch DC, Gupta A. . Ophthalmic cyclosporine use in ocular GVHD. Cornea. 2006; 25:635–8.

29. Yuan J, Zhai JJ, Chen JQ. . Preparation of 0.05% FK506 sus-pension eyedrops and its pharmacokinetics after topical ocular administration. J Ocul Pharmacol Ther. 2009; 25:345–50.

30. Thomson AW. Interspecies comparison of the immunosuppressive efficacy and safety of FK 506. Transplant Proc. 1990; 22:100–5.
Figure 1.
Slit lamp photographs with fluorescein staining in chronic ocular graft-versus-host disease patient with severe dry eye syndrome. (A) Prior to treatment, definite corneal lesions were noted (OSDI: 42 points, TBUT: 0 sec, staining score: 5 points, Schirmer I test: 0 mm). (B) After 28 days of treatment with topical 0.03% tacrolimus eye drops, the previous lesions showed im-provements (OSDI: 32 points, TBUT: 2 sec, staining score: 2 points, Schirmer I test: 2 mm). OSDI = ocular surface disease index; TBUT = tear film break up time.

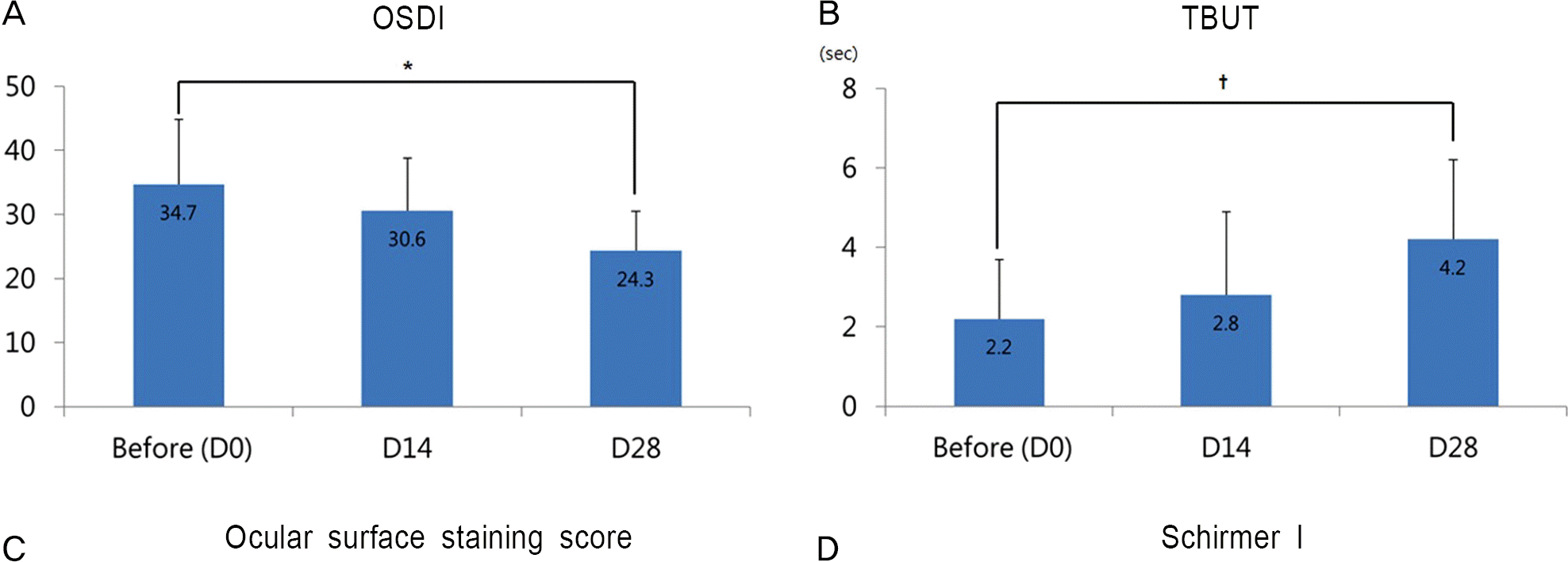
Figure 2.
Changes before (D0) and after 14 (D14), 28 (D28) days of treatment with 0.03% tacrolimus eye drops in refractory dry eye associated with graft-versus-host disease. (A) OSDI. (B) TBUT. (C) Ocular surface staining score, and (D) Schirmer I test. The bars represent the mean of each value. The error bars indicate the standard deviation of the mean. OSDI = ocular surface disease index; TBUT = tear film break up time. * p= 0.024; † p=0.015; ‡ p=0.031.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download