Abstract
Purpose
To evaluate outcomes of intravitreal aflibercept in cases resistant to bevacizumab and ranibizumab in neovascular age-related macular degeneration.
Methods
Twenty patients with neovascular age-related macular generation who were resistant to treatment with bevacizumab and ranibizumab were evaluated. After switching to aflibercept the best corrected visual acuity (BCVA) and central retinal thick-ness (CRT) were compared at baseline and at 1 month after injection. Additionally, changes in the intraretinal fluid, subretinal flu-id and pigment epithelial detachment were evaluated.
Results
The mean BCVA was 0.83 ± 0.56 log MAR and the mean CRT was 294.20 ± 12.99 μ m before aflibercept treatment. After switching to aflibercept the mean BCVA was 0.86 ± 0.61 log MAR with no statistical difference ( p = 0.406) and the mean CRT was decreased to 232.45 ± 12.05 μm (p = 0.011). After 1 month of aflibercept injections, a reduction of intraretinal fluid in 4 eyes (80%), reduction of subretinal fluid in 11 eyes (78.6%) and reduction of pigment epithelial detachment in 5 eyes (50%) were observed. Increases in fluid or new lesions were not observed.
Go to : 
References
1. Friedman DS, O'Colmain BJ, Muñoz B. . Prevalence of age-re-lated macular degeneration in the United States. Arch Ophthalmol. 2004; 122:564–72.

2. Augood C, Fletcher A, Bentham G. . Methods for a pop-ulation-based study of the prevalence of and risk factors for age-re-lated maculopathy and macular degeneration in elderly European populations: the EUREYE study. Ophthalmic Epidemiol. 2004; 11:117–29.

3. Resnikoff S, Pascolini D, Etya'ale D. . Global data on visual impairment in the year 2002. Bull World Health Organ. 2004; 82:844–51.
4. Au Eong KG. Age-related macular degeneration: an emerging challenge for eye care and public health professionals in the Asia Pacific region. Ann Acad Med Singapore. 2006; 35:133–5.
5. Frampton JE. Ranibizumab: a review of its use in the treatment of neovascular age-related macular degeneration. Drugs Aging. 2013; 30:331–58.

6. Schouten JS, La Heij EC, Webers CA. . A systematic review on the effect of bevacizumab in exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2009; 247:1–11.

7. Heier JS, Brown DM, Chong V. . Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012; 119:2537–48.

8. Browning DJ, Kaiser PK, Rosenfeld PJ, Stewart MW. Aflibercept for age-related macular degeneration: a game-changer or quiet ad-dition? Am J Ophthalmol. 2012; 154:222–6.
9. Holash J, Davis S, Papadopoulos N. . VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002; 99:11393–8.

10. Papadopoulos N, Martin J, Ruan Q. . Binding and neutraliza-tion of vascular endothelial growth factor (VEGF) and related li-gands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012; 15:171–85.

11. Dixon JA, Oliver SC, Olson JL, Mandava N. VEGF Trap-Eye for the treatment of neovascular age-related macular degeneration. Expert Opin Investig Drugs. 2009; 18:1573–80.

12. Stewart MW, Rosenfeld PJ. Predicted biological activity of intra-vitreal VEGF Trap. Br J Ophthalmol. 2008; 92:667–8.

13. Chappelow AV, Kaiser PK. Neovascular age-related macular de-generation: potential therapies. Drugs. 2008; 68:1029–36.
14. Rudge JS, Thurston G, Davis S. . VEGF trap as a novel anti-angiogenic treatment currently in clinical trials for cancer and eye diseases, and VelociGene- based discovery of the next generation of angiogenesis targets. Cold Spring Harb Symp Quant Biol. 2005; 70:411–8.
15. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004; 25:581–611.

16. Ferrara N, Damico L, Shams N. . Development of ranibizu-mab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006; 26:859–70.

17. Binder S. Loss of reactivity in intravitreal anti-VEGF therapy: ta-chyphylaxis or tolerance? Br J Ophthalmol. 2012; 96:1–2.
18. Gasperini JL, Fawzi AA, Khondkaryan A. . Bevacizumab and ranibizumab tachyphylaxis in the treatment of choroidal neo- vascularisation. Br J Ophthalmol. 2012; 96:14–20.
19. Cho H, Shah CP, Weber M. . Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J Ophthalmol. 2013; 97:1032–5.

20. Ho VY, Yeh S, Olsen TW. . Short-term outcomes of aflibercept for neovascular age-related macular degeneration in eyes pre-viously treated with other vascular endothelial growth factor inhibitors. Am J Ophthalmol. 2013; 156:23–8.e2.

21. Moore DJ, Hussain AA, Marshall J. Age-related variation in the hydraulic conductivity of Bruch's membrane. Invest Ophthalmol Vis Sci. 1995; 36:1290–7.
22. Hageman GS, Luthert PJ, Victor Chong NH. . An integrated hypothesis that considers drusen as biomarkers of immune-medi-ated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001; 20:705–32.

23. Oh H, Takagi H, Takagi C. . The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1999; 40:1891–8.
Go to : 
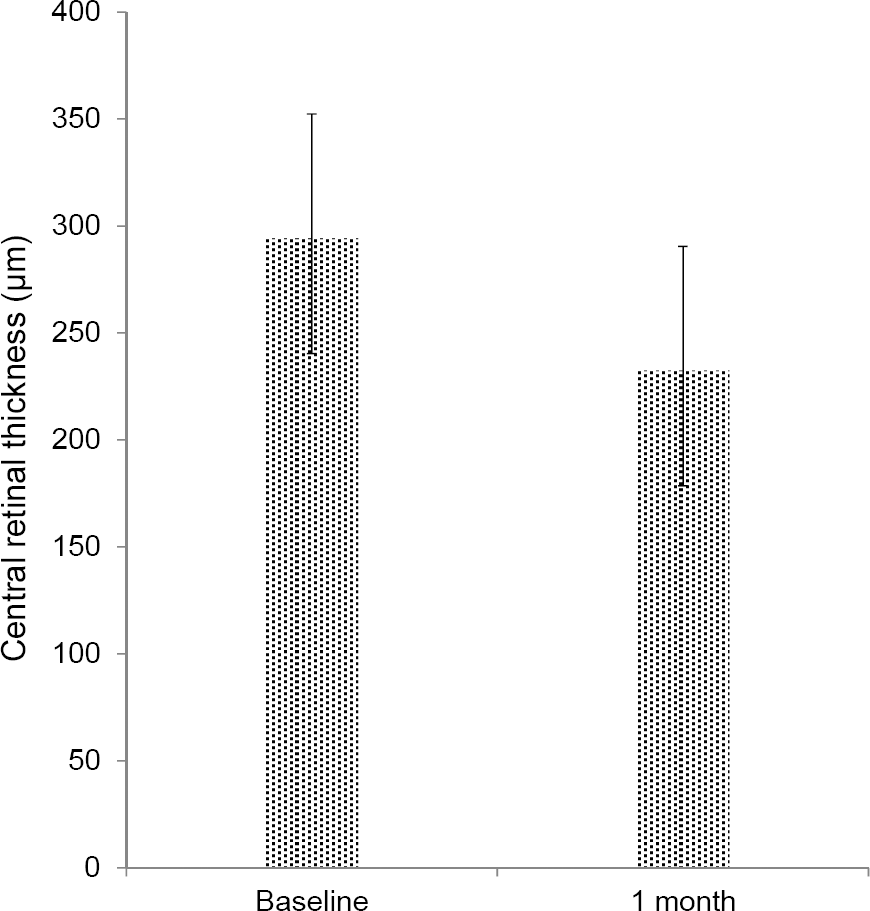 | Figure 1.Change in central retinal thickness from baseline to 1 month after intravitreal aflibercept injection who were re-sistant to treatment with bevacizumab and ranibizumab in neo-vascular age-related macular degeneration (Paired t-test, p = 0.011). |
 | Figure 2.A patient treated with 10th intravitreal bevacizumab and 9th intravitreal ranibizumab injection. Optical coherence tomog-raphy (OCT) showed persistent intraretinal (IRF), subretinal fluid (SRF) and pigment epithelial detachment (PED) (A). 1 month af-ter the first intravitreal aflibercept injection, OCT shows complete absorption of IRF, SRF with decreased PED (B). |
Table 1.
Dermographic features and patient’s characteristics at baseline
Table 2.
Comparison of visual acuity, central macular thickness between at baseline and at 1 month after intravitreal aflibercept in-jection
| Baseline | At 1 month | p-value∗ | |
|---|---|---|---|
| Visual acuity (log MAR) | 0.83 ± 0.56 | 0.86 ± 0.61 | 0.406 |
| Central retinal thickness (μ m) | 294.20 ± 12.99 | 232.45 ± 12.05 | 0.011 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download