Abstract
Purpose
To evaluate the peripapillary retinal nerve fiber layer (pRNFL) thickness and macular ganglion cell-inner plexiform lay-er (mGCIPL) thickness in eyes with resolved diabetic macular edema (DME).
Methods
Twenty eyes of diabetic retinopathy patients with resolved DME (DME group) after treatment, and 20 eyes of diabetic retinopathy patients without DME (no-DME group) were included in this study. The pRNFL thickness, mGCIPL thickness and central macular thickness (CMT) were measured using spectral-domain optical coherence tomography (SD-OCT). Analyses were performed to determine the correlation between the different thicknesses and the visual function.
Results
No significant difference in mean CMT was observed between the DME and no-DME groups. Average pRNFL thick-ness in the DME group was thicker than in the no-DME group ( p = 0.003). Average mGCIPL thickness in the DME group was thinner than in the no-DME group ( p = 0.030). Final visual acuity was significantly correlated with average mGCIPL thickness and minimum mGCIPL thickness, but not pRNFL thickness and CMT in the DME group.
References
1. Diabetic Retinopathy Clinical Research Network. Browning DJ, Glassman AR. . Relationship between optical coherence to-mography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007; 114:525–36.
2. Alasil T, Keane PA, Updike JF. . Relationship between optical coherence tomography retinal parameters and visual acuity in dia-betic macular edema. Ophthalmology. 2010; 117:2379–86.

3. Uji A, Murakami T, Nishijima K. . Association between hyper-reflective foci in the outer retina, status of photoreceptor layer, and visual acuity in diabetic macular edema. Am J Ophthalmol. 2012; 153:710–7. 717.e1.

4. Sakamoto A, Nishijima K, Kita M. . Association between fo-veal photoreceptor status and visual acuity after resolution of dia-betic macular edema by pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2009; 247:1325–30.

5. Takahashi H, Goto T, Shoji T. . Diabetes-associated retinal nerve fiber damage evaluated with scanning laser polarimetry. Am J Ophthalmol. 2006; 142:88–94.

6. Sugimoto M, Sasoh M, Ido M. . Detection of early diabetic change with optical coherence tomography in type 2 diabetes mel-litus patients without retinopathy. Ophthalmologica. 2005; 219:379–85.

7. van Dijk HW, Verbraak FD, Kok PH. . Decreased retinal gan-glion cell layer thickness in patients with type 1 diabetes. Invest Ophthalmol Vis Sci. 2010; 51:3660–5.

8. Pierro L, Gagliardi M, Iuliano L. . Retinal nerve fiber layer thickness reproducibility using seven different OCT instruments. Invest Ophthalmol Vis Sci. 2012; 53:5912–20.

9. Mwanza JC, Durbin MK, Budenz DL. . Profile and predictors of normal ganglion cell-inner plexiform layer thickness measured with frequency-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011; 52:7872–9.

10. Massin P, Audren F, Haouchine B. . Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: preliminary results of a prospective controlled trial. Ophthalmology. 2004; 111:218–24.
11. Yanoff M, Fine BS, Brucker AJ, Eagle RC Jr. Pathology of human cystoid macular edema. Surv Ophthalmol 1984;28 Suppl. 505–11.

12. Shin HJ, Lee SH, Chung H, Kim HC. Association between photo-receptor integrity and visual outcome in diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2012; 250:61–70.

13. Murakami T, Nishijima K, Akagi T. . Segmentational analysis of retinal thickness after vitrectomy in diabetic macular edema. Invest Ophthalmol Vis Sci. 2012; 53:6668–74.

14. Pelosini L, Hull CC, Boyce JF. . Optical coherence tomog-raphy may be used to predict visual acuity in patients with macular edema. Invest Ophthalmol Vis Sci. 2011; 52:2741–8.

15. Sung MS, Yoon JH, Park SW. Diagnostic validity of macular gan-glion cell-inner plexiform layer thickness deviation map algorithm using cirrus HD-OCT in preperimetric and early glaucoma. J Glaucoma. 2014; 23:e144–51.

16. Park KA, Park DY, Oh SY. Analysis of spectral-domain optical co-herence tomography measurements in amblyopia: a pilot study. Br J Ophthalmol. 2011; 95:1700–6.

17. Zhang L, Ino-ue M, Dong K, Yamamoto M. Retrograde axonal transport impairment of large- and medium-sized retinal ganglion cells in diabetic rat. Curr Eye Res. 2000; 20:131–6.

18. Park HY, Kim IT, Park CK. Early diabetic changes in the nerve fi-bre layer at the macula detected by spectral domain optical coher-ence tomography. Br J Ophthalmol. 2011; 95:1223–8.

19. Kim JT, Lee JK, Moon NJ, Cho HK. Analysis of the optic nerve head and RNFL thickness using optical coherence tomography in diabetes. J Korean Ophthalmol Soc. 2008; 49:935–41.

20. Hwang DJ, Lee EJ, Lee SY. . Effect of diabetic macular edema on peripapillary retinal nerve fiber layer thickness profiles. Invest Ophthalmol Vis Sci. 2014; 55:4213–9.

21. Takayama K, Hangai M, Durbin M. . A novel method to detect local ganglion cell loss in early glaucoma using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53:6904–13.

22. Howell SJ, Mekhail MN, Azem R. . Degeneration of retinal ganglion cells in diabetic dogs and mice: relationship to glycemic control and retinal capillary degeneration. Mol Vis. 2013; 19:1413–21.
23. Chung HS, Harris A, Halter PJ. . Regional differences in retinal vascular reactivity. Invest Ophthalmol Vis Sci. 1999; 40:2448–53.
24. Jonas JB, Naumann GO. Parapapillary retinal vessel diameter in normal and glaucoma eyes. II. Correlations. Invest Ophthalmol Vis Sci. 1989; 30:1604–11.
25. Königsreuther KA, Jonas JB. Optic disc morphology in diabetes mellitus. Graefes Arch Clin Exp Ophthalmol. 1995; 233:200–4.

26. Kanamori A, Escano MF, Eno A. . Evaluation of the effect of aging on retinal nerve fiber layer thickness measured by optical co-herence tomography. Ophthalmologica. 2003; 217:273–8.

27. Tilton RG, Chang KC, LeJeune WS. . Role for nitric oxide in the hyperpermeability and hemodynamic changes induced by in-travenous VEGF. Invest Ophthalmol Vis Sci. 1999; 40:689–96.
28. Foxton RH, Finkelstein A, Vijay S. . VEGF-A is necessary and sufficient for retinal neuroprotection in models of experimental glaucoma. Am J Pathol. 2013; 182:1379–90.

29. Tatlipinar S, Dinç UA, Yenerel NM, Görgün E. Short-term effects of a single intravitreal bevacizumab injection on retinal vessel calibre. Clin Exp Optom. 2012; 95:94–8.

30. Goel N, Kumar V, Ghosh B. Ischemic maculopathy following in-travitreal bevacizumab for refractory diabetic macular edema. Int Ophthalmol. 2011; 31:39–42.

Figure 1.
Scatter plot of end-point BCVA (log MAR) versus average pRNFL thickness, temporal pRNFL thickness, average GCIPL thickness, minimum GCIPL thickness and average CMT in DME eyes (n = 20). (A) Correlation between average pRNFL thickness and end-point BCVA. (B) Correlation between temporal pRNFL thickness and end-point BCVA. (C) Correlation between average GCIPL thickness and end-point BCVA. (D) Correlation between minimum GCIPL thickness and end-point BCVA. (E) Correlation between average CMT and end-point BCVA. BCVA = best corrected visual acuity; pRNFL = peripapillary retinal nerve fiber lay-er; GCIPL = Ganglion cell-inner plexiform layer; CMT = central macular thickness; DME = diabetic macular edema.
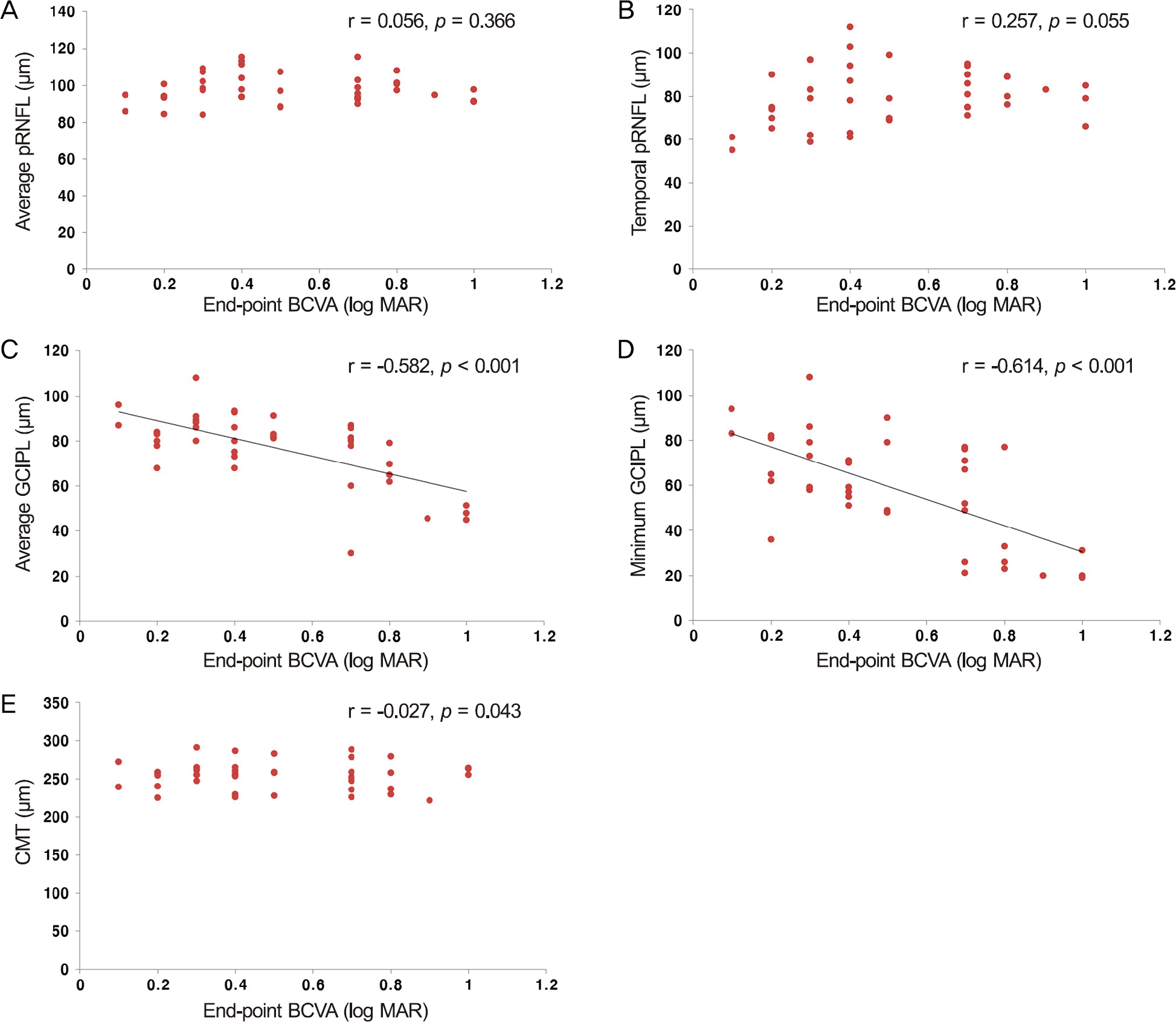
Table 1.
Clinical characteristics of eyes with DME group and no-DME group
Values are presented as mean ± SD unless otherwise indicated. DME = diabetic macular edema; M = male; F = female; BCVA = best corrected visual acuity; SE = spherical equivalent; DM = diabetes mellitus; NPDR = non proliferative diabetic retinopathy; PDR = proliferative diabetic retinopathy; B = bevacizumab; TA = triamcinolone acetonide; CMT = central macular thickness; N/A = not available.
Table 2.
Comparison of pRNFL parameter between eyes with DME group and no-DME group measured by Cirrus OCT
Table 3.
Comparison of mGCIPL parameter between eyes with DME group and no-DME group measured by Cirrus OCT
Table 4.
Comparison of mGCIPL parameter and CMT between PRP group and no-PRP group measured by Cirrus OCT




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download