Abstract
Purpose
To examine the changes in meibomian glands associated with aging in a normal Korean population and to estimate the differences between the upper and lower eyelid in each age group.
Methods
We performed meibography on adult subjects using an infrared charge-coupled device (CCD) camera. Each eyelid was scored based on the loss of meibomian glands, and the meiboscores of the upper and lower eyelids were summed to obtain a score for each eye. Meiboscores were evaluated according to age, sex, and upper and lower eyelid meiboscores in each age group.
Results
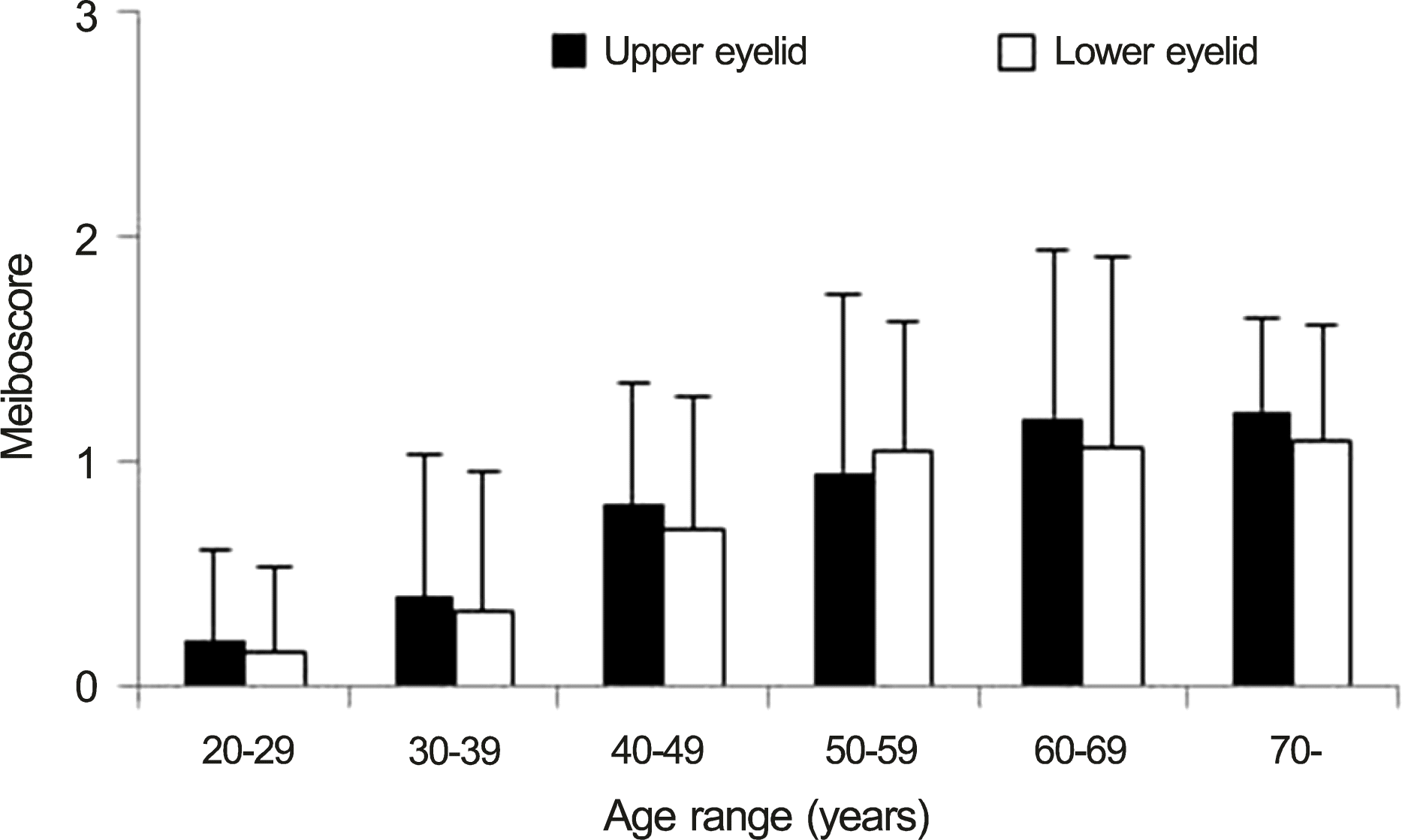
One hundred seventeen eyes of 117 people were enrolled in this study. The study subjects had an average age of 50.4 ± 19.1 years (range, 20-92; male, 56; female, 61). There was a significant positive correlation between age and total meiboscore, upper and lower eyelid meiboscore (r = 0.578, p < 0.001; r = 0.550, p < 0.001; r = 0.524, p < 0.001). There were no significant differences in the meiboscores of the upper and lower eyelids in any age group, though meiboscores were significantly higher since 40 year-old group than 20 year-old group (p < 0.001, p < 0.001, p < 0.001, p < 0.001).
Go to : 
References
1. MISHIMA S, MAURICE DM. The oily layer of the tear film and evaporation from the corneal surface. Exp Eye Res. 1961; 1:39–45.

2. Nelson JD, Shimazaki J, Benitez-del-Castillo JM. . The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011; 52:1930–7.

3. Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf. 2003; 1:107–26.

4. Mathers WD. Ocular evaporation in meibomian gland dysfunction and dry eye. Ophthalmology. 1993; 100:347–51.

5. Cho JH, Ahn Y. Assessment of meibomian gland dysfunction and comparison of the results of BUT and Schirmer test according to meibomian gland state. J Korean Ophthalmol Soc. 2000; 41:1875–82.
6. Mathers WD, Daley T, Verdick R. Video imaging of the meibomian gland. Arch Ophthalmol. 1994; 112:448–9.

7. Nichols JJ, Berntsen DA, Mitchell GL, Nichols KK. An assessment of grading scales for meibography images. Cornea. 2005; 24:382–8.

8. Tomlinson A, Bron AJ, Korb DR. . The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011; 52:2006–49.

9. Ibrahim OM, Matsumoto Y, Dogru M. . The efficacy, sensitivity, and specificity of in vivo laser confocal microscopy in the diagnosis of meibomian gland dysfunction. Ophthalmology. 2010; 117:665–72.

10. Hwang HS, Park CW, Joo CK. Novel noncontact meibography with anterior segment optical coherence tomography: Hosik meibography. Cornea. 2013; 32:40–3.
11. Srinivasan S, Menzies K, Sorbara L, Jones L. Infrared imaging of meibomian gland structure using a novel keratograph. Optom Vis Sci. 2012; 89:788–94.

12. Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology. 2008; 115:911–5.

13. Tapie R. Biomicroscopic study of the glands of meibomius. Ann Ocul. 1977; 210:637–48.
14. Yokoi N, Komuro A, Yamada H. . A newly developed vid-eo-meibography system featuring a newly designed probe. Jpn J Ophthalmol. 2007; 51:53–6.

15. Jester JV, Rife L, Nii D. . In vivo biomicroscopy and photography of meibomian glands in a rabbit model of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 1982; 22:660–7.
16. Den S, Shimizu K, Ikeda T. . Association between meibomian gland changes and aging, sex, or tear function. Cornea. 2006; 25:651–5.

17. Norn M. Expressibility of meibomian secretion. Relation to age, lipid precorneal film, scales, foam, hair and pigmentation. Acta Ophthalmol (Copenh). 1987; 65:137–42.
Go to : 
 | Figure 1.Photos demonstrating representative cases of each meibography grade. Meibomian glands was scored using meiboscore: (A) Grade 0, no meibomian gland dropout. (B) Grade 1, meibomian gland dropout <1/3 of total meibomian glands. (C) Grade 2, meibomian gland dropout in more than 1/3 and less than 2/3 of the total meibomian glands. (D) Grade 3, meibomian gland dropout >2/3 of the total meibomian glands. Arrows indicate meibomian dropout. Meiboscores for the upper and lower eyelids were summed to obtain a score for each eye. |
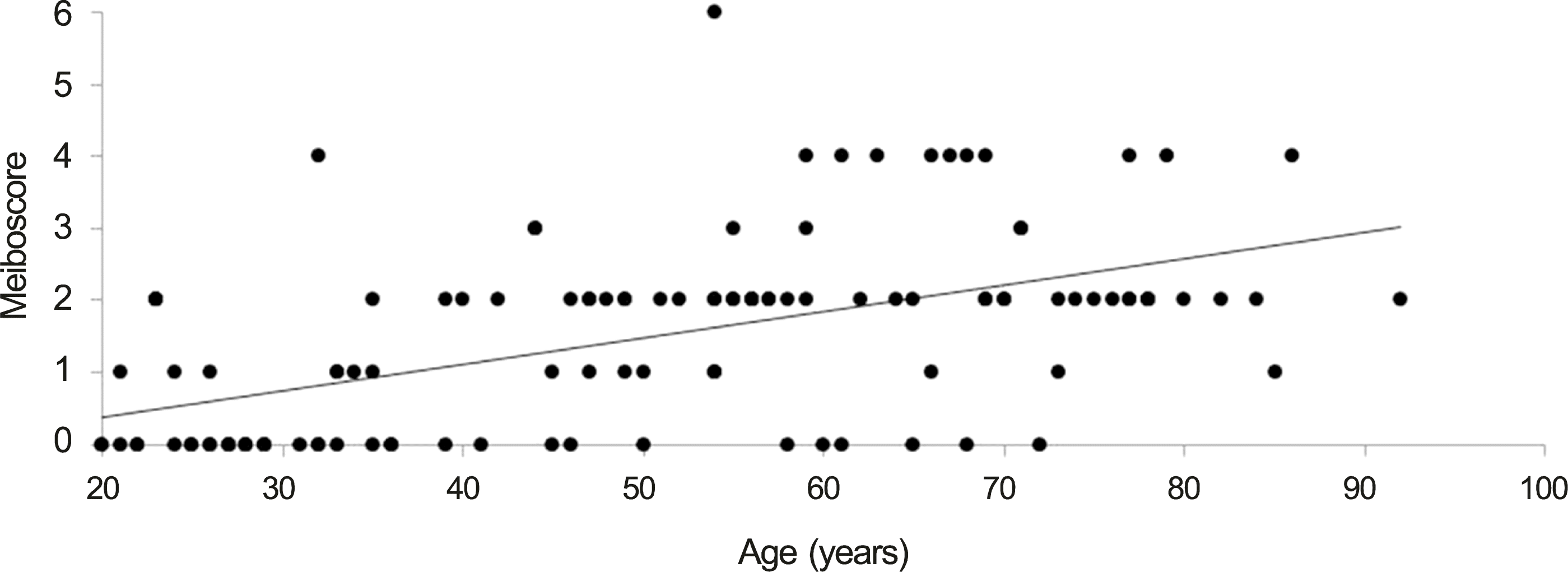 | Figure 2.Distribution and change of total meiboscore. There was a significant positive correlation between age and total meiboscore (r = 0.578; p < 0.001, Spearman’s correlation analysis). |
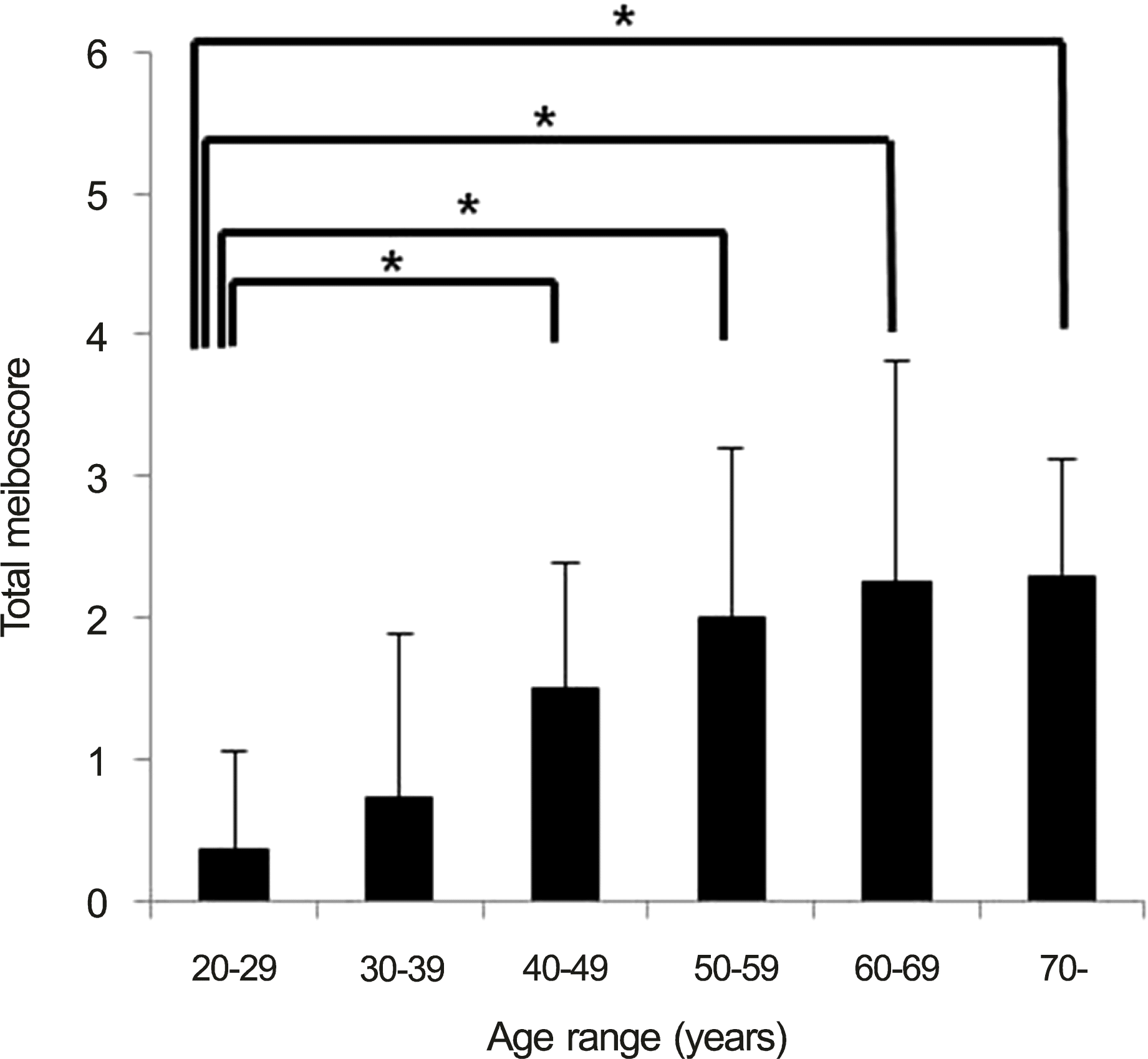 | Figure 3.The graph showing meiboscore changes according to age. There were significant difference between the groups (p < 0.001, Kruskall-Wallis test). After 40 years of age, meiboscores were significantly higher than the 20 to 29 year-old group (30-39, p = 0.276; 40-49, p < 0.001; 50-59, p < 0.001; 60-69, p < 0.001; 70-, p < 0.01, Mann-Whitney * p < 0.05, Mann-Whitney U-test between 20-29 U-test). year-old group and another age groups. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download