Abstract
Purpose
To determine the effect of intravitreal bevacizumab injection before panretinal photocoagulation (PRP) in clinically sig-nificant macular edema (CSME) patients.
Methods
A total of 91 eyes (70 patients) having severe nonproliferative diabetic retinopathy with CSME requiring PRP were en-rolled in the present study; the medical records were retrospectively reviewed and analyzed. The eyes were divided into the reg-ular PRP group (51 eyes) and PRP with preinjection of bevacizumab (1.25 mg) group (combination group, 40 eyes) and compared. Best corrected visual acuity (BCVA) and central macular thickness (CMT) at pretreatment and 1, 3, 6, and 12 months after PRP was evaluated.
Results
BCVA (logarithm of the minimum angle of resolution, Snellen visual acuity in parentheses) at pretreatment and 1, 3, 6, and 12 months after PRP was 0.24 (0.575), 0.27 (0.537), 0.28 (0.525), 0.28 (0.525), and 0.30 (0.501) ( p = 0.13, 0.15, 0.56 and 0.79) in the regular PRP group and 0.32 (0.479), 0.25 (0.562), 0.26 (0.549), 0.27 (0.537), and 0.36 (0.436) ( p = 0.02, 0.04, 0.02 and 0.13) in the combination group, respectively. CMT (µm) at pretreatment and 1, 3, 6, and 12 months after PRP was 257.66, 285.16, 282.21, 289.65, and 309.85 ( p = 0.00, 0.00, 0.00 and 0.00) in the regular PRP group and 349.39, 312.17, 331.15. 353.30, and 333.55 ( p = 0.04, 0.94, 0.79 and 0.06) in the combination group, respectively.
Go to : 
References
1. Massin P, Bandello F, Garweg JG. . Safety and efficacy of rani-bizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010; 33:2399–405.
2. Soheilian M, Ramezani A, Obudi A. . Randomized trial of in-travitreal bevacizumab alone or combined with triamcinolone ver-sus macular photocoagulation in diabetic macular edema. Ophthalmology. 2009; 116:1142–50.

3. Photocoagulation treatment of proliferative diabetic retinopathy Clinical application of Diabetic Retinopathy Study (DRS) find-ings, DRS Report Number 8. The Diabetic Retinopathy Study Research Group. Ophthalmology. 1981; 88:583–600.
4. McDonald HR, Schatz H. Visual loss following panretinal photo-coagulation for proliferative diabetic retinopathy. Ophthalmology. 1985; 92:388–93.

5. Mason JO 3rd, Yunker JJ, Vail R, McGwin G Jr. Intravitreal bev-acizumab (Avastin) prevention of panretinal photocoagulation- induced complications in patients with severe proliferative diabetic retinopathy. Retina. 2008; 28:1319–24.
6. Early photocoagulation for diabetic retinopathy ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991; 98:(5 Suppl). 766–85.
7. Tae KS, Moon YS, Chin HS. Short term clinical courses after pan-retinal photocoagulation treatment in diabetic retinopathy patients. J Korean Ophthalmol Soc. 2003; 44:1996–2003.
8. Cho WB, Oh SB, Moon JW, Kim HC. Panretinal photocoagulation combined with intravitreal bevacizumab in high-risk proliferative diabetic retinopathy. Retina. 2009; 29:516–22.

9. Choi JH, Lee SJ, Choi KS. Long-term effect of panretinal photo-coagulation combined with intravitreal bevacizumab in high-risk proliferative diabetic retinopathy. J Korean Ophthalmol Soc. 2010; 51:842–8.

10. Avery RL. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina. 2006; 26:352–4.

11. Cho WB, Moon JW, Kim HC. Intravitreal triamcinolone and bev-acizumab as adjunctive treatments to panretinal photocoagulation in diabetic retinopathy. Br J Ophthalmol. 2010; 94:858–63.

12. McDonald HR, Schatz H. Macular edema following panretinal photocoagulation. Retina. 1985; 5:5–10.

13. Nonaka A, Kiryu J, Tsujikawa A. . Inflammatory response af-ter scatter laser photocoagulation in nonphotocoagulated retina. Invest Ophthalmol Vis Sci. 2002; 43:1204–9.
14. Brooks HL Jr, Caballero S Jr, Newell CK. . Vitreous levels of vascular endothelial growth factor and stromal-derived factor 1 in patients with diabetic retinopathy and cystoid macular edema be-fore and after intraocular injection of triamcinolone. Arch Ophthalmol. 2004; 122:1801–7.
15. Haritoglou C, Kook D, Neubauer A. . Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006; 26:999–1005.

16. Jeon S, Lee WK. Effect of intravitreal bevacizumab on diabetic macular edema with hard exudates. Clin Ophthalmol. 2014; 8:1479–86.
17. Song JH, Lee JJ, Lee SJ. Comparison of the short-term effects of intravitreal triamcinolone acetonide and bevacizumab injection for diabetic macular edema. Korean J Ophthalmol. 2011; 25:156–60.

18. Diabetic Retinopathy Clinical Research Network. Scott IU, Edwards AR. . A phase II randomized clinical trial of intra-vitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007; 114:1860–7.
19. Kook D, Wolf A, Kreutzer T. . Long-term effect of intravitreal bevacizumab (avastin) in patients with chronic diffuse diabetic macular edema. Retina. 2008; 28:1053–60.

20. Arevalo JF, Sanchez JG, Fromow-Guerra J. . Comparison of two doses of primary intravitreal bevacizumab (Avastin) for dif-fuse diabetic macular edema: results from the Pan-American Collaborative Retina Study Group (PACORES) at 12-month fol-low-up. Graefes Arch Clin Exp Ophthalmol. 2009; 247:735–43.

Go to : 
 | Figure 1.Photographs of fundus and optical coherence tomography. (A, B) Baseline evaluation of PRP only group (arrow: scan di-rection). (C, D) Baseline evaluation of AVI + PRP combination group (arrow: scan direction). PRP = panretinal photocoagulation; AVI = anti-vascular endothelial growth factor injection. |
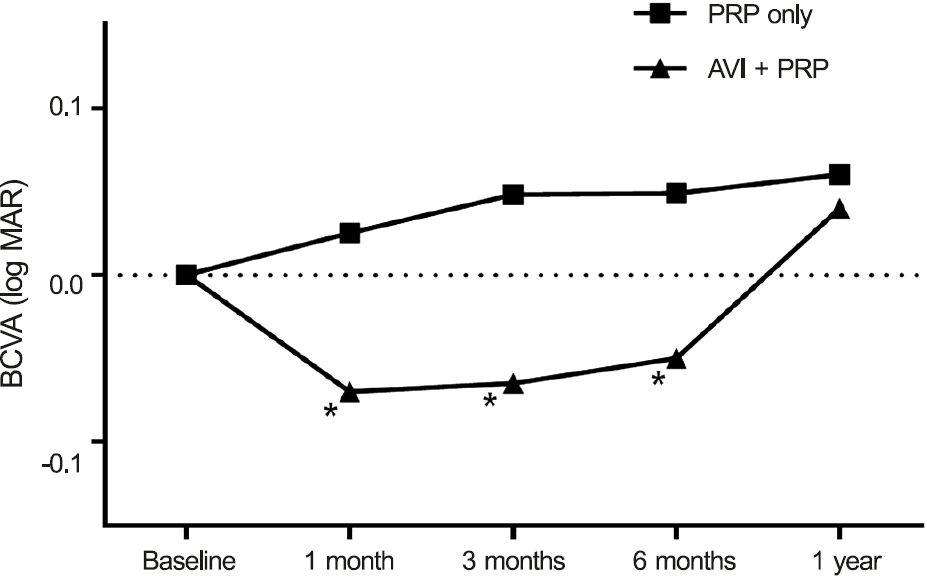 | Figure 2.Changes in best corrected visual acuity (BCVA) af-ter treatment in the two groups. Combination group shows sig-nificant improvement of BCVA for 6 months after treatment compared to baseline. log MAR = logarithm of minimum an-gle of resolution; PRP = panretinal photocoagulation; AVI = anti-vascular endothelial growth factor injection. * Compare to before injection, mean value reaches statistical significance ( p < 0.05). Tested by paired t-test. |
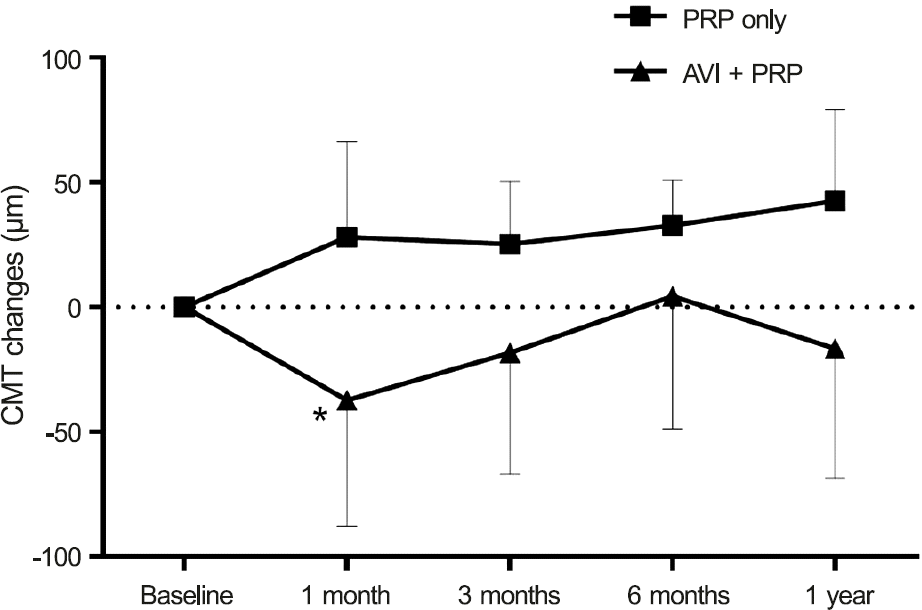 | Figure 3.Changes in central macular thickness (CMT) after treatment in the two groups. Combination group shows sig-nificant reduction of CMT at 1 month after treatment com-pared to baseline. PRP = panretinal photocoagulation; AVI = anti-vascular endothelial growth factor injection. * Compare to before injection, mean value reaches statistical significance ( p < 0.05). Tested by paired t-test. |
Table 1.
Basic characteristics of two groups
| Variables | PRP only | AVI + PRP | p-value |
|---|---|---|---|
| Sex (M:F) | 20:21 | 15:14 | 0.601∗ |
| Age (years) | 58.65 ± 10.22 | 53.70 ± 11.10 | 0.030† |
| BCVA (log MAR) | 0.24 ± 0.25 | 0.32 ± 0.31 | 0.134† |
| IOP (mm Hg) | 14.46 ± 2.99 | 15.43 ± 2.94 | 0.264† |
| CMT (μ m) | 257.66 ± 54.31 | 349.39 ± 110.25 | 0.011† |
| Duration of DM (years) | 12.22 ± 7.86 | 13.86 ± 5.37 | 0.293† |
| HbA1c (%) | 7.92 ± 1.49 | 8.66 ± 2.22 | 0.125† |
| Height (cm) | 160.41 ± 8.40 | 158.59 ± 7.59 | 0.472† |
| Weight (kg) | 66.48 ± 21.38 | 58.85 ± 10.45 | 0.163† |
| Hb (g/dL) | 12.01 ± 1.69 | 12.30 ± 2.29 | 0.553† |
| WBC (x10.e3/μ L) | 7.87 ± 4.92 | 11.67 ± 15.39 | 0.184† |
| PLT (x10.e3/μ L) | 220.53 ± 40.15 | 242.55 ± 51.95 | 0.055† |
| Hct (%) | 35.04 ± 5.05 | 35.91 ± 6.64 | 0.544† |
| TG (mg/dL) | 193.09 ± 106.65 | 193.02 ± 108.06 | 0.998† |
| Total cholesterol (mg/dL) | 185.75 ± 47.21 | 179.88 ± 41.32 | 0.573† |
| HDL (mg/dL) | 40.01 ± 12.90 | 43.44 ± 11.98 | 0.246† |
| BUN (mg/dL) | 18.66 ± 6.28 | 22.47 ± 13.60 | 0.130† |
| Cr (mg/dL) | 1.04 ± 0.32 | 1.35 ± 1.13 | 0.125† |
| GFR (mL/min/1.73m2) | 66.83 ± 36.13 | 53.94 ± 36.15 | 0.115† |
Values are presented as mean ± SD unless otherwise indicated. PRP = panretinal photocoagulation; AVI = anti-vascular endothelial growth factor injection; BCVA = best corrected visual acuity; log MAR = logarithm of minimum angle of resolution; IOP = intraocular pressure; CMT = central macular thickness; DM = diabetes mellitus; HbA1c = glycosylated haemoglobin; Hb = hemoglobin; WBC = white blood cell; PLT = platelet; Hct = hematocrit; TG = triglyceride; HDL = high density lipoprotein; BUN = blood urea nitrogen; Cr = creatine; GFR = glomerular filtration rate.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download