Abstract
Purpose
To evaluate the effectiveness of a dexamethasone intravitreal implant (Ozurdex®) in the treatment of diabetic macular edema refractory to combined treatment of bevacizumab and triamcinolone.
Methods
We reviewed 9 eyes of 9 patients with diabetic macular edema treated with dexamethasone intravitreal implant. The patients were included in the study if presenting with refractory diabetic macular edema of more than 3 months despite combined treatment of intravitreal bevacizumab injection with posterior subtenon triamcinolone injection or intravitreal triamcinolone injection. We assessed the best-corrected visual acuity (BCVA) and central macular thickness (CMT) using optical coherence to-mography at initial visit and 1, 3 and 4 months.
Results
The mean follow-up was 6.7 ± 2.2 months. The baseline mean BCVA was 0.81 ± 0.47 logarithm of the minimum angle of resolution (log MAR), which improved to 0.61 ± 0.37 log MAR ( p = 0.017), 0.57 ± 0.38 log MAR ( p = 0.011) and 0.62 ± 0.36 log MAR ( p = 0.027) at 1 month, 3 months and 4 months, respectively. The baseline mean CMT was 558.0 ± 110.32 μ m and de-creased to 325 ± 64.21 μ m ( p = 0.008) and 300.22 ± 59.46 μ m ( p = 0.008) at 1 month and 3 months, respectively, then increased to 468.44 ± 150.85 μ m ( p = 0.058) at 4 months after injection.
Conclusions
Dexamethasone intravitreal implant showed short-term efficacy in the treatment of diabetic macular edema re-fractory to combined treatment of bevacizumab and triamcinolone and produced significant improvements in BCVA and CMT until 3 months after injection. The CMT then increased, but BCVA was sustained until the fourth month.
Go to : 
References
1. Photocoagulation for diabetic macular edema Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985; 103:1796–806.
2. Antcliff RJ, Marshall J. The pathogenesis of edema in diabetic maculopathy. Semin Ophthalmol. 1999; 14:223–32.

3. Nguyen QD, Shah SM, Khwaja AA. . Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2010; 117:2146–51.

4. Diabetic Retinopathy Clinical Research Network. Scott IU, Edwards AR. . A phase II randomized clinical trial of intra-vitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007; 114:1860–7.
5. Haritoglou C, Kook D, Neubauer A. . Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006; 26:999–1005.

6. Massin P, Audren F, Haouchine B. . Intravitreal triamcinolone acetonide for diabeitc diffuse macular edema: preliminary results of a prospective controlled trial. Ophthalmology. 2004; 111:218–24. discussion 224-5.
7. Jung JW, Nam DH, Shyn KH. The complications after intravitreal injection of triamcinolone acetonide. J Korean Ophthalmol Soc. 2007; 48:55–62.
8. Kuppermann BD, Blumenkranz MS, Haller JA. . Dexamethasone DDS Phase II Study Group: randomized controlled study of an in-travitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch Ophthalmol. 2007; 125:309–17.
9. Haller JA, Bandello F, Belfort R Jr. . Randomized, sham-con-trolled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010; 117:1134–46.

10. Zarranz-Ventura J, Carreño E, Johnston RL. . Multicenter study of intravitreal dexamethasone implant in noninfectious uvei-tis: indications, outcomes, and reinjection frequency. Am J Ophthalmol. 2014; 158:1136–45.e5.

11. Gillies MC, Lim LL, Campain A. . A randomized clinical trial of intravitreal bevacizumab versusi dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology. 2014; 121:2473–81.
12. Lazic R, Lukic M, Boras I. . Treatment of anti-vascular endo-thelial growth factor-resistant diabetic macular edema with dex-amethasone intravitreal implant. Retina. 2014; 34:719–24.

13. Pacella E, Vestri AR, Muscella R. . Preliminary results of an in-travitreal dexamethasone implant (Ozurdex[R]) in patients with persistent diabetic macular edema. Clin Ophthalmol. 2013; 7:1423–8.
14. Zalewski D, Raczyń ska D, Raczyń ska K. Five-month observation of persistent diabetic macular edema after intravitreal injection of Ozurdex implant. Mediators Inflamm. 2014; 2014:364143.

15. Dutra Medeiros M, Postorino M, Navarro R. . Dexamethasone intravitreal implant for treatment of patients with persistent dia-betic macular edema. Ophthalmologica. 2014; 231:141–6.

16. Zucchiatti I, Lattanzio R, Querques G. . Intravitreal dex-amethasone implant in patients with persistent diabetic macular edema. Ophthalmologica. 2012; 228:117–22.

17. Rishi P, Rishi E, Kuniyal L, Mathur G. Short-term results of intra-vitreal dexamethasone implant (OZURDEX [R]) in treatment of recalcitrant diabetic macular edema: a case series. Oman J Ophthalmol. 2012; 5:79–82.
18. Funatsu H, Noma H, Mimura T. . Association of vitreous in-flammatory factors with diabetic macular edema. Ophthalmology. 2009; 116:73–9.

19. Lavinsky D, Cardillo JA, Melo LA Jr. . Randomized clinical trial evaluating mETDRS versus normal or high-density micro-pulse photocoagulation for diabetic macular edema. Invest Ophthalmol Vis Sci. 2011; 52:4314–23.

20. Writing Committee for the Diabetic Retinopathy Clinical Research Network. Fong DS, Strauber SF. . Comparison of the modified Early Treatment Diabetic Retinopathy Study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol. 2007; 125:469–80.
21. Guyer DR, D’Amico DJ, Smith CW. Subretinal fibrosis after laser photocoagulation for diabetic macular edema. Am J Ophthalmol. 1992; 113:652–6.
22. Schatz H, Madeira D, McDonald HR, Johnson RN. Progressive en-largement of laser scars following grid laser photocoagulation for diffuse diabetic macular edema. Arch Ophthalmol. 1991; 109:1549–51.

23. Lewis H, Schachat AP, Haimann MH. . Choroidal neo-vascularization after laser photocoagulation for diabetic macular edema. Ophthalmology. 1990; 97:503–10.

24. Morgan CM, Schatz H. Atrophic creep of the retinal pigment epi-thelium after focal macular photocoagulation. Ophthalmology. 1989; 96:96–103.

25. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004; 25:581–611.

26. Funatsu H, Yamashita H, Ikeda T. . Vitreous levels of inter-leukin-6 and vascular endothelial growth factor are related to dia-betic macular edema. Ophthalmology. 2003; 110:1690–6.

27. Haritoglou C, Kook D, Neubauer A. . Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006; 26:999–1005.

28. Fischer S, Renz D, Schaper W, Karliczek GF. In vitro effects of dexamethasone on hypoxia-induced hyperpermeability and ex-pression of vascular endothelial growth factor. Eur J Pharmacol. 2001; 411:231–43.

29. Bandi N, Kompella UB. Budesonide reduces vascular endothelial growth factor secretion and expression in airway (Calu-1) and al-veolar (A549) epithelial cells. Eur J Pharmacol. 2001; 425:109–16.

30. Gillies MC, Sutter FK, Simpson JM. . Intravitreal tri-amcinolone for refractory diabetic macular edema: two-year re-sults of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology. 2006; 113:1533–8.
31. Shimura M, Nakazawa T, Yasuda K. . Comparative therapy evaluation of intravitreal bevacizumab and triamcinolone aceto-nide on persistent diffuse diabetic macular edema. Am J Ophthalmol. 2008; 145:854–61.

32. Paccola L, Costa RA, Folgosa MS. . Intravitreal triamcinolone versus bevacizumab for treatment of refractory diabetic macular oedema (IBEME study). Br J Ophthalmol. 2008; 92:76–80.

33. Ahmadieh H, Ramezani A, Shoeibi N. . Intravitreal bev-acizumab with or without triamcinolone for refractory diabetic macular edema: a placebocontrolled, randomized clinical trial. Graefes Arch Clin Exp Ophthalmol. 2008; 246:483–9.

34. Yolcu U, Sobaci G. The effect of combined treatment of bev-acizumab and triamcinolone for diabetic macular edema refractory to previous intravitreal mono-injections. Int Ophthalmol. 2014; Nov. [Epub ahead of print].

35. Kim HD, Kang KD, Choi KS. . Combined therapy with intra-vitreal bevacizumab and posterior subtenon triamcinolone aceto-nide injection in diabetic macular oedema. Acta Ophthalmol. 2014; 92:e589–90.

36. Edelman JL. Differentiating intraocular glucocorticoids. Ophthal- mologica. 2010; 224Suppl 1. 25–30.

37. Chang-Lin JE, Attar M, Acheampong AA. . Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone in-travitreal implant. Invest Ophthalmol Vis Sci. 2011; 52:80–6.

Go to : 
 | Figure 3.Optical coherence tomography images of 2 patients with persistent diabetic macular edema nonresponsive to pre-vious combined treatment of bevacizumab and triamcinolone, before combined treatment (A, F), at baseline (B, G) and after treatment (C-E, H-J) with dexamethasone intravitreal implant. Patient 1 shows complete resolution of the macular edema, Patient 2 shows nearly complete resolution of the macular edema. |
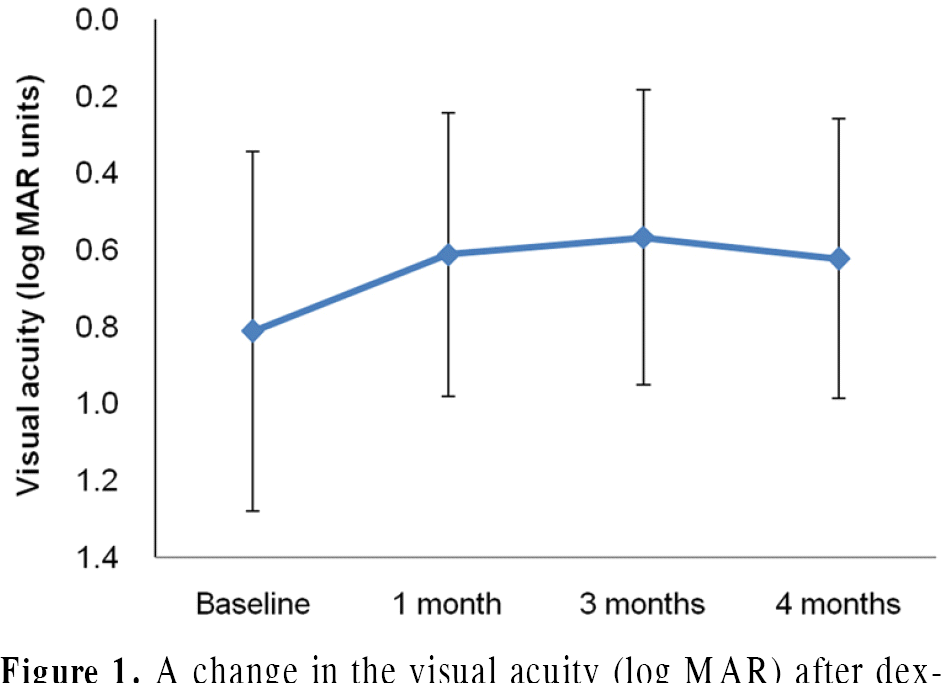 | Figure 1.A change in the visual acuity (log MAR) after dex-amethasone intravitreal implant injection. Mean visual acuity prior to injection was 0.81 ± 0.47. Visual acuity improved to 0.61 ± 0.37, 0.57 ± 0.38, 0.62 ± 0.36 in 1, 3 and 4 months after injection. |
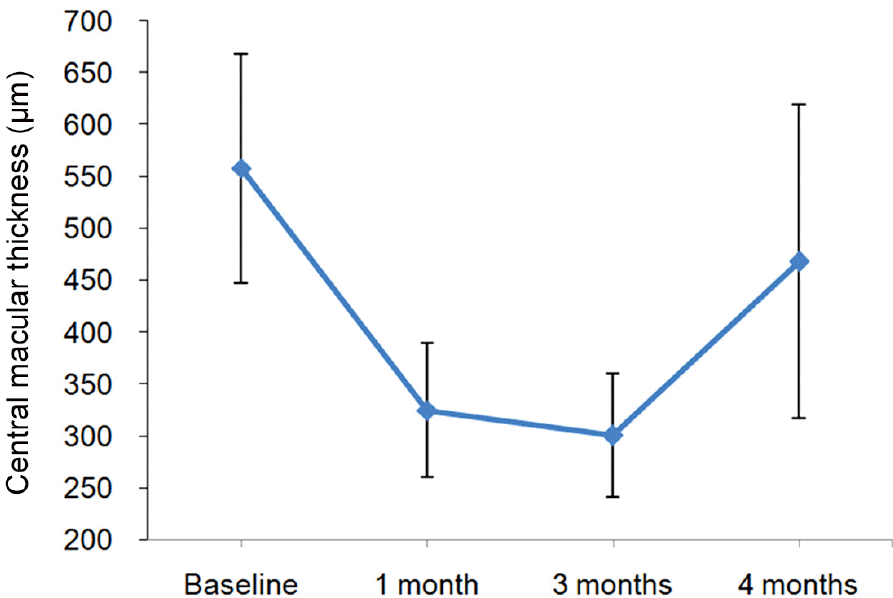 | Figure 2.A change in the central macular thickness after dex-amethasone intravitreal implant injection. Mean central mac-ular thickness prior to injection was 558.0 ± 110.32 μ m. Central macular thickness decreased to 325 ± 64.21 μ m and 300.22 ± 59.46 μ m, 1 and 3 months after injection. Then it in-creased to 468.44 ± 150.85 μ m in 4 months after injection. |
Table 1.
Demographic characteristics of the patients
| Variable | Value |
|---|---|
| Patients (eyes) | 9 (9) |
| Age (years) | 63.4 ± 9.51 |
| Male:female | 5:4 |
| Follow-up time (months) | 6.7 ± 2.2 |
| Baseline visual acuity (log MAR) | 0.81 ± 0.47 |
| Baseline CMT (μ m) | 558.0 ± 110.32 |
Table 2.
Previous medical history of the patients
| Patient No. | Focal laser | Number of IVB | Number of IVB + PST | Number of IVB + IVT |
|---|---|---|---|---|
| 1 | Yes | 3 | 2 | 0 |
| 2 | Yes | 5 | 2 | 0 |
| 3 | Yes | 2 | 1 | 2 |
| 4 | Yes | 2 | 1 | 2 |
| 5 | Yes | 8 | 2 | 0 |
| 6 | Yes | 2 | 1 | 1 |
| 7 | Yes | 2 | 2 | 2 |
| 8 | Yes | 2 | 0 | 1 |
| 9 | Yes | 8 | 0 | 1 |
Table 3.
Mean changes in visual acuity and central macular thickness after dexamethasone intravitreal implant injection




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download